پلوتونيوم
 | |||||||||||||||
| الپلوتونيوم | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| التآصلات | انظر تآصلات الپلوتونيوم | ||||||||||||||
| المظهر | أبيض فضي، يفقد لمعانه ويتحول للرمادي الداكن عند التعرض للهواء | ||||||||||||||
| الپلوتونيوم في الجدول الدوري | |||||||||||||||
| |||||||||||||||
| الرقم الذري (Z) | 94 | ||||||||||||||
| المجموعة | n/a | ||||||||||||||
| الدورة | period 7 | ||||||||||||||
| المستوى الفرعي | f-block | ||||||||||||||
| التوزيع الإلكتروني | [Rn] 5f6 7s2 | ||||||||||||||
| الإلكترونات بالغلاف | 2, 8, 18, 32, 24, 8, 2 | ||||||||||||||
| الخصائص الطبيعية | |||||||||||||||
| الطور at د.ح.ض.ق | صلب | ||||||||||||||
| نقطة الانصهار | 912.5 K (639.4 °س، 1182.9 °F) | ||||||||||||||
| نقطة الغليان | 3505 K (3228 °س، 5842 °ف) | ||||||||||||||
| الكثافة (بالقرب من د.ح.غ.) | 19.816 ج/سم³ | ||||||||||||||
| حين يكون سائلاً (عند ن.إ.) | 16.63 ج/سم³ | ||||||||||||||
| حرارة الانصهار | 2.82 kJ/mol | ||||||||||||||
| حرارة التبخر | 333.5 kJ/mol | ||||||||||||||
| السعة الحرارية المولية | 35.5 J/(mol·K) | ||||||||||||||
ضغط البخار
| |||||||||||||||
| الخصائص الذرية | |||||||||||||||
| حالات الأكسدة | 8, 7, 6, 5, 4, 3, 2, 1 | ||||||||||||||
| الكهرسلبية | مقياس پاولنگ: 1.28 | ||||||||||||||
| طاقات التأين |
| ||||||||||||||
| نصف القطر الذري | empirical: 159 pm | ||||||||||||||
| نصف قطر التكافؤ | 187±1 pm | ||||||||||||||
| خصائص أخرى | |||||||||||||||
| البنية البلورية | monoclinic | ||||||||||||||
| سرعة الصوت | 2260 م/ث | ||||||||||||||
| قضيب رفيع | 6.74 W/(m·K) | ||||||||||||||
| التمدد الحراري | 46.7 µm/(m⋅K) (عند 25 °س) | ||||||||||||||
| المقاومة الكهربائية | 1.460 µΩ⋅m (at 0 °C) | ||||||||||||||
| الترتيب المغناطيسي | مغناطيس مساير | ||||||||||||||
| معامل يونگ | 96 GPa | ||||||||||||||
| معامل القص | 43 GPa | ||||||||||||||
| نسبة پواسون | 0.21 | ||||||||||||||
| رقم كاس | 7440-07-5 | ||||||||||||||
| التاريخ | |||||||||||||||
| التسمية | على اسم الكوكب القزم پلوتو، والذي سمي على اسم إله العالم السفلي الكلاسيكي پلوتو | ||||||||||||||
| الاكتشاف | گلن ت. سيبورگ، آرثر وال، جوسف و. كندي، إدوين مكميلان (1940–1) | ||||||||||||||
| نظائر الالپلوتونيوم | |||||||||||||||
| قالب:جدول نظائر الپلوتونيوم غير موجود | |||||||||||||||
الپلوتونيوم Plutonium، هو عنصر كيميائي ترابي مشع رمزه الكيميائي وعدده الذري 94. وهو فلز أكتيني ذو لون رمادي-فضي يفقد لمعانه عند التعرض للهواء، ولوناً باهتاً عن تأكسده. يتفاع مع الكربون، الهالوجينات، النيتروجين، السليكون، والهيدروجين. عند تعرضه للهواء الرطب، يشكل أكاسيد وهيدريات تتمدد لأكبر من 70% من حجمها، والتي تتحول بدورها إلى مسحوق تلقائي الاشتعال. وهو مادة مشعة ويمكن أن تتراكم في العظام، مما يجعل التعامل مع الپلوتونيوم خطراً.
Plutonium was first synthetically produced and isolated in late 1940 and early 1941, by a deuteron bombardment of uranium-238 in the 1.5-متر (60 in) cyclotron at the University of California, Berkeley. First, neptunium-238 (half-life 2.1 days) was synthesized, which subsequently beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium had been named after the planet Uranus and neptunium after the planet Neptune, element 94 was named after Pluto, which at the time was considered to be a planet as well. Wartime secrecy prevented the University of California team from publishing its discovery until 1948.
Plutonium is the element with the highest atomic number to occur in nature. Trace quantities arise in natural uranium-238 deposits when uranium-238 captures neutrons emitted by decay of other uranium-238 atoms. Plutonium is much more common on Earth since 1945 as a product of neutron capture and beta decay, where some of the neutrons released by the fission process convert uranium-238 nuclei into plutonium-239.
The quantity of isotopes in the decay chains at a certain time are calculated with the Bateman equation. Both plutonium-239 and plutonium-241 are fissile, meaning that they can sustain a nuclear chain reaction, leading to applications in nuclear weapons and nuclear reactors. Plutonium-240 exhibits a high rate of spontaneous fission, raising the neutron flux of any sample containing it. The presence of plutonium-240 limits a plutonium sample's usability for weapons or its quality as reactor fuel, and the percentage of plutonium-240 determines its grade (weapons-grade, fuel-grade, or reactor-grade). Plutonium-238 has a half-life of 87.7 years and emits alpha particles. It is a heat source in radioisotope thermoelectric generators, which are used to power some spacecraft. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized reactors.
Producing plutonium in useful quantities for the first time was a major part of the Manhattan Project during World War II that developed the first atomic bombs. The Fat Man bombs used in the Trinity nuclear test in July 1945, and in the bombing of Nagasaki in August 1945, had plutonium cores. Human radiation experiments studying plutonium were conducted without informed consent, and several criticality accidents, some lethal, occurred after the war. Disposal of plutonium waste from nuclear power plants and dismantled nuclear weapons built during the Cold War is a nuclear-proliferation and environmental concern. Other sources of plutonium in the environment are fallout from numerous above-ground nuclear tests, now banned.
الخصائص
الخصائص الطبيعية
Plutonium, like most metals, has a bright silvery appearance at first, much like nickel, but it oxidizes very quickly to a dull gray, although yellow and olive green are also reported.[1][2] At room temperature plutonium is in its α (alpha) form. This, the most common structural form of the element (allotrope), is about as hard and brittle as gray cast iron unless it is alloyed with other metals to make it soft and ductile. Unlike most metals, it is not a good conductor of heat or electricity. It has a low melting point (640 °C) and an unusually high boiling point (3,228 °C).[1]
Alpha decay, the release of a high-energy helium nucleus, is the most common form of radioactive decay for plutonium.[3] A 5 kg mass of 239Pu contains about 12.5×1024 atoms. With a half-life of 24,100 years, about 11.5×1012 of its atoms decay each second by emitting a 5.157 MeV alpha particle. This amounts to 9.68 watts of power. Heat produced by the deceleration of these alpha particles makes it warm to the touch.[4][5]
Resistivity is a measure of how strongly a material opposes the flow of electric current. The resistivity of plutonium at room temperature is very high for a metal, and it gets even higher with lower temperatures, which is unusual for metals.[6] This trend continues down to 100 K, below which resistivity rapidly decreases for fresh samples.[6] Resistivity then begins to increase with time at around 20 K due to radiation damage, with the rate dictated by the isotopic composition of the sample.[6]
Because of self-irradiation, a sample of plutonium fatigues throughout its crystal structure, meaning the ordered arrangement of its atoms becomes disrupted by radiation with time.[7] Self-irradiation can also lead to annealing which counteracts some of the fatigue effects as temperature increases above 100 K.[8]
Unlike most materials, plutonium increases in density when it melts, by 2.5%, but the liquid metal exhibits a linear decrease in density with temperature.[6] Near the melting point, the liquid plutonium has very high viscosity and surface tension compared to other metals.[7]
التآصلات

Plutonium normally has six allotropes and forms a seventh (zeta, ζ) at high temperature within a limited pressure range.[9] These allotropes, which are different structural modifications or forms of an element, have very similar internal energies but significantly varying densities and crystal structures. This makes plutonium very sensitive to changes in temperature, pressure, or chemistry, and allows for dramatic volume changes following phase transitions from one allotropic form to another.[7] The densities of the different allotropes vary from 16.00 g/cm3 to 19.86 g/cm3.[10]
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. For example, the α form exists at room temperature in unalloyed plutonium. It has machining characteristics similar to cast iron but changes to the plastic and malleable β (beta) form at slightly higher temperatures.[11] The reasons for the complicated phase diagram are not entirely understood. The α form has a low-symmetry monoclinic structure, hence its brittleness, strength, compressibility, and poor thermal conductivity.[9]
Plutonium in the δ (delta) form normally exists in the 310 °C to 452 °C range but is stable at room temperature when alloyed with a small percentage of gallium, aluminium, or cerium, enhancing workability and allowing it to be welded.[11] The δ form has more typical metallic character, and is roughly as strong and malleable as aluminium.[9] In fission weapons, the explosive shock waves used to compress a plutonium core will also cause a transition from the usual δ phase plutonium to the denser α form, significantly helping to achieve supercriticality.[12] The ε phase, the highest temperature solid allotrope, exhibits anomalously high atomic self-diffusion compared to other elements.[7]
الانشطار النووي

Plutonium is a radioactive actinide metal whose isotope, plutonium-239, is one of the three primary fissile isotopes (uranium-233 and uranium-235 are the other two); plutonium-241 is also highly fissile. To be considered fissile, an isotope's atomic nucleus must be able to break apart or fission when struck by a slow moving neutron and to release enough additional neutrons to sustain the nuclear chain reaction by splitting further nuclei.[13]
Pure plutonium-239 may have a multiplication factor (keff) larger than one, which means that if the metal is present in sufficient quantity and with an appropriate geometry (e.g., a sphere of sufficient size), it can form a critical mass.[14] During fission, a fraction of the nuclear binding energy, which holds a nucleus together, is released as a large amount of electromagnetic and kinetic energy (much of the latter being quickly converted to thermal energy). Fission of a kilogram of plutonium-239 can produce an explosion equivalent to 21،000 tons of TNT (88،000 GJ). It is this energy that makes plutonium-239 useful in nuclear weapons and reactors.[4]
The presence of the isotope plutonium-240 in a sample limits its nuclear bomb potential, as plutonium-240 has a relatively high spontaneous fission rate (~440 fissions per second per gram—over 1,000 neutrons per second per gram),[15] raising the background neutron levels and thus increasing the risk of predetonation.[16] Plutonium is identified as either weapons-grade, fuel-grade, or reactor-grade based on the percentage of plutonium-240 that it contains. Weapons-grade plutonium contains less than 7% plutonium-240. Fuel-grade plutonium contains from 7% to less than 19%, and power reactor-grade contains 19% or more plutonium-240. Supergrade plutonium, with less than 4% of plutonium-240, is used in U.S. Navy weapons stored in proximity to ship and submarine crews, due to its lower radioactivity.[17] The isotope plutonium-238 is not fissile but can undergo nuclear fission easily with fast neutrons as well as alpha decay.[4]
النظائر والتخليق النووي
Twenty radioactive isotopes of plutonium have been characterized. The longest-lived are plutonium-244, with a half-life of 80.8 million years, plutonium-242, with a half-life of 373,300 years, and plutonium-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight metastable states, though all have half-lives less than one second.[3]
The known isotopes of plutonium range in mass number from 228 to 247. The primary decay modes of isotopes with mass numbers lower than the most stable isotope, plutonium-244, are spontaneous fission and alpha emission, mostly forming uranium (92 protons) and neptunium (93 protons) isotopes as decay products (neglecting the wide range of daughter nuclei created by fission processes). The primary decay mode for isotopes with mass numbers higher than plutonium-244 is beta emission, mostly forming americium (95 protons) isotopes as decay products. Plutonium-241 is the parent isotope of the neptunium decay series, decaying to americium-241 via beta emission.[3][18]
Plutonium-238 and 239 are the most widely synthesized isotopes.[4] Plutonium-239 is synthesized via the following reaction using uranium (U) and neutrons (n) via beta decay (β−) with neptunium (Np) as an intermediate:[19]
Neutrons from the fission of uranium-235 are captured by uranium-238 nuclei to form uranium-239; a beta decay converts a neutron into a proton to form neptunium-239 (half-life 2.36 days) and another beta decay forms plutonium-239.[20] Egon Bretscher working on the British Tube Alloys project predicted this reaction theoretically in 1940.[21]
Plutonium-238 is synthesized by bombarding uranium-238 with deuterons (D, the nuclei of heavy hydrogen) in the following reaction:[22]
In this process, a deuteron hitting uranium-238 produces two neutrons and neptunium-238, which spontaneously decays by emitting negative beta particles to form plutonium-238.[23]
حرارة الاضمحلال وخصائص الانشطار
Plutonium isotopes undergo radioactive decay, which produces decay heat. Different isotopes produce different amounts of heat per mass. The decay heat is usually listed as watt/kilogram, or milliwatt/gram. In larger pieces of plutonium (e.g. a weapon pit) and inadequate heat removal the resulting self-heating may be significant.
| Isotope | Decay mode | Half-life (years) | Decay heat (W/kg) | Spontaneous fission neutrons (1/(g·s)) | Comment |
|---|---|---|---|---|---|
| 238Pu | alpha to 234U | 87.74 | 560 | 2600 | Very high decay heat. Even in small amounts can cause significant self-heating. Used on its own in radioisotope thermoelectric generators. |
| 239Pu | alpha to 235U | 24100 | 1.9 | 0.022 | The principal fissile isotope in use. |
| 240Pu | alpha to 236U, spontaneous fission | 6560 | 6.8 | 910 | The principal impurity in samples of the 239Pu isotope. The plutonium grade is usually listed as percentage of 240Pu. High spontaneous fission hinders use in nuclear weapons. |
| 241Pu | beta-minus, to 241Am | 14.4 | 4.2 | 0.049 | Decays to americium-241; its buildup presents a radiation hazard in older samples. |
| 242Pu | alpha to 238U | 376000 | 0.1 | 1700 | 242Pu decays to 238U through alpha decay; will also decay by spontaneous fission. |
المركبات والكيمياء
At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized.[25] The element displays four common ionic oxidation states in aqueous solution and one rare one:[10]
- Pu(III), as Pu3+ (blue lavender)
- Pu(IV), as Pu4+ (yellow brown)
- Pu(V), as PuO+2 (light pink)[note 1]
- Pu(VI), as PuO2+2 (pink orange)
- Pu(VII), as PuO3−5 (green)—the heptavalent ion is rare.
The color shown by plutonium solutions depends on both the oxidation state and the nature of the acid anion.[27] It is the acid anion that influences the degree of complexing—how atoms connect to a central atom—of the plutonium species. Additionally, the formal +2 oxidation state of plutonium is known in the complex [K(2.2.2-cryptand)] [PuIICp″3], Cp″ = C5H3(SiMe3)2.[28]
A +8 oxidation state is possible as well in the volatile tetroxide PuO 4.[29] Though it readily decomposes via a reduction mechanism similar to FeO 4, PuO 4 can be stabilized in alkaline solutions and chloroform.[30][29]
Metallic plutonium is produced by reacting plutonium tetrafluoride with barium, calcium or lithium at 1200 °C.[31] It is attacked by acids, oxygen, and steam but not by alkalis and dissolves easily in concentrated hydrochloric, hydroiodic and perchloric acids.[32] Molten metal must be kept in a vacuum or an inert atmosphere to avoid reaction with air.[11] At 135 °C the metal will ignite in air and will explode if placed in carbon tetrachloride.[33]

Plutonium is a reactive metal. In moist air or moist argon, the metal oxidizes rapidly, producing a mixture of oxides and hydrides.[1] If the metal is exposed long enough to a limited amount of water vapor, a powdery surface coating of PuO2 is formed.[1] Also formed is plutonium hydride but an excess of water vapor forms only PuO2.[32]
Plutonium shows enormous, and reversible, reaction rates with pure hydrogen, forming plutonium hydride.[7] It also reacts readily with oxygen, forming PuO and PuO2 as well as intermediate oxides; plutonium oxide fills 40% more volume than plutonium metal. The metal reacts with the halogens, giving rise to compounds with the general formula PuX3 where X can be F, Cl, Br or I and PuF4 is also seen. The following oxyhalides are observed: PuOCl, PuOBr and PuOI. It will react with carbon to form PuC, nitrogen to form PuN and silicon to form PuSi2.[10][33]
Powders of plutonium, its hydrides and certain oxides like Pu2O3 are pyrophoric, meaning they can ignite spontaneously at ambient temperature and are therefore handled in an inert, dry atmosphere of nitrogen or argon. Bulk plutonium ignites only when heated above 400 °C. Pu2O3 spontaneously heats up and transforms into PuO2, which is stable in dry air, but reacts with water vapor when heated.[34]
Crucibles used to contain plutonium need to be able to withstand its strongly reducing properties. Refractory metals such as tantalum and tungsten along with the more stable oxides, borides, carbides, nitrides and silicides can tolerate this. Melting in an electric arc furnace can be used to produce small ingots of the metal without the need for a crucible.[11]
Cerium is used as a chemical simulant of plutonium for development of containment, extraction, and other technologies.[35]
البنية الإلكترونية
Plutonium is an element in which the 5f electrons are the transition border between delocalized and localized; it is therefore considered one of the most complex elements.[36] The anomalous behavior of plutonium is caused by its electronic structure. The energy difference between the 6d and 5f subshells is very low. The size of the 5f shell is just enough to allow the electrons to form bonds within the lattice, on the very boundary between localized and bonding behavior. The proximity of energy levels leads to multiple low-energy electron configurations with near equal energy levels. This leads to competing 5fn7s2 and 5fn−16d17s2 configurations, which causes the complexity of its chemical behavior. The highly directional nature of 5f orbitals is responsible for directional covalent bonds in molecules and complexes of plutonium.[7]
السبائك
Plutonium can form alloys and intermediate compounds with most other metals. Exceptions include lithium, sodium, potassium, rubidium and caesium of the alkali metals; and magnesium, calcium, strontium, and barium of the alkaline earth metals; and europium and ytterbium of the rare earth metals.[32] Partial exceptions include the refractory metals chromium, molybdenum, niobium, tantalum, and tungsten, which are soluble in liquid plutonium, but insoluble or only slightly soluble in solid plutonium.[32] Gallium, aluminium, americium, scandium and cerium can stabilize the δ phase of plutonium for room temperature. Silicon, indium, zinc and zirconium allow formation of metastable δ state when rapidly cooled. High amounts of hafnium, holmium and thallium also allows some retention of the δ phase at room temperature. Neptunium is the only element that can stabilize the α phase at higher temperatures.[7]
Plutonium alloys can be produced by adding a metal to molten plutonium. If the alloying metal is sufficiently reductive, plutonium can be added in the form of oxides or halides. The δ phase plutonium–gallium and plutonium–aluminium alloys are produced by adding plutonium(III) fluoride to molten gallium or aluminium, which has the advantage of avoiding dealing directly with the highly reactive plutonium metal.[37]
- Plutonium–gallium is used for stabilizing the δ phase of plutonium, avoiding the α-phase and α–δ related issues. Its main use is in pits of implosion nuclear weapons.[38]
- Plutonium–aluminium is an alternative to the Pu–Ga alloy. It was the original element considered for δ phase stabilization, but its tendency to react with the alpha particles and release neutrons reduces its usability for nuclear weapon pits. Plutonium–aluminium alloy can be also used as a component of nuclear fuel.[39]
- Plutonium–gallium–cobalt alloy (PuCoGa5) is an unconventional superconductor, showing superconductivity below 18.5 K, an order of magnitude higher than the highest between heavy fermion systems, and has large critical current.[36][40]
- Plutonium–zirconium alloy can be used as nuclear fuel.[41]
- Plutonium–cerium and plutonium–cerium–cobalt alloys are used as nuclear fuels.[42]
- Plutonium–uranium, with about 15–30 mol.% plutonium, can be used as a nuclear fuel for fast breeder reactors. Its pyrophoric nature and high susceptibility to corrosion to the point of self-igniting or disintegrating after exposure to air require alloying with other components. Addition of aluminium, carbon or copper does not improve disintegration rates markedly, zirconium and iron alloys have better corrosion resistance but they disintegrate in several months in air as well. Addition of titanium and/or zirconium significantly increases the melting point of the alloy.[43]
- Plutonium–uranium–titanium and plutonium–uranium–zirconium were investigated for use as nuclear fuels. The addition of the third element increases corrosion resistance, reduces flammability, and improves ductility, fabricability, strength, and thermal expansion. Plutonium–uranium–molybdenum has the best corrosion resistance, forming a protective film of oxides, but titanium and zirconium are preferred for physics reasons.[43]
- Thorium–uranium–plutonium was investigated as a nuclear fuel for fast breeder reactors.[43]
التواجد

Trace amounts of plutonium-238, plutonium-239, plutonium-240, and plutonium-244 can be found in nature. Small traces of plutonium-239, a few parts per trillion, and its decay products are naturally found in some concentrated ores of uranium,[44] such as the natural nuclear fission reactor in Oklo, Gabon.[45] The ratio of plutonium-239 to uranium at the Cigar Lake Mine uranium deposit ranges from 2.4×10−12 to 44×10−12.[46] These trace amounts of 239Pu originate in the following fashion: on rare occasions, 238U undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy. When one of these neutrons strikes the nucleus of another 238U atom, it is absorbed by the atom, which becomes 239U. With a relatively short half-life, 239U decays to 239Np, which decays into 239Pu.[47][48] Finally, exceedingly small amounts of plutonium-238, attributed to the extremely rare double beta decay of uranium-238, have been found in natural uranium samples.[49]
Due to its relatively long half-life of about 80 million years, it was suggested that plutonium-244 occurs naturally as a primordial nuclide, but early reports of its detection could not be confirmed.[50] However, its long half-life ensured its circulation across the solar system before its extinction,[51] and indeed, evidence of the spontaneous fission of extinct 244Pu has been found in meteorites.[52] The former presence of 244Pu in the early Solar System has been confirmed, since it manifests itself today as an excess of its daughters, either 232Th (from the alpha decay pathway) or xenon isotopes (from its spontaneous fission). The latter are generally more useful, because the chemistries of thorium and plutonium are rather similar (both are predominantly tetravalent) and hence an excess of thorium would not be strong evidence that some of it was formed as a plutonium daughter.[53] 244Pu has the longest half-life of all transuranic nuclides and is produced only in the r-process in supernovae and colliding neutron stars; when nuclei are ejected from these events at high speed to reach Earth, 244Pu alone among transuranic nuclides has a long enough half-life to survive the journey, and hence tiny traces of live interstellar 244Pu have been found in the deep sea floor. Because 240Pu also occurs in the decay chain of 244Pu, it must thus also be present in secular equilibrium, albeit in even tinier quantities.[54]
Minute traces of plutonium are usually found in the human body due to the 550 atmospheric and underwater nuclear tests that have been carried out, and to a small number of major nuclear accidents. Most atmospheric and underwater nuclear testing was stopped by the Limited Test Ban Treaty in 1963, which was signed and ratified by the United States, the United Kingdom, the Soviet Union, and other nations. Continued atmospheric nuclear weapons testing since 1963 by non-treaty nations included those by China (atomic bomb test above the Gobi Desert in 1964, hydrogen bomb test in 1967, and follow-on tests), and France (tests as recently as the 1990s). Because it is deliberately manufactured for nuclear weapons and nuclear reactors, plutonium-239 is the most abundant isotope of plutonium by far.[33]
التاريخ
الاكتشاف
Enrico Fermi and a team of scientists at the University of Rome reported that they had discovered element 94 in 1934.[55] Fermi called the element hesperium and mentioned it in his Nobel Lecture in 1938.[56] The sample was actually a mixture of barium, krypton, and other elements, but this was not known at the time.[57] Nuclear fission was discovered in Germany in 1938 by Otto Hahn and Fritz Strassmann. The mechanism of fission was then theoretically explained by Lise Meitner and Otto Frisch.[58]


Plutonium (specifically, plutonium-238) was first produced, isolated and then chemically identified between December 1940 and February 1941 by Glenn T. Seaborg, Edwin McMillan, Emilio Segrè, Joseph W. Kennedy, and Arthur Wahl by deuteron bombardment of uranium in the 60-بوصة (150 cm) cyclotron at the Berkeley Radiation Laboratory at the University of California, Berkeley.[59][60][61] Neptunium-238 was created directly by the bombardment but decayed by beta emission with a half-life of a little over two days, which indicated the formation of element 94.[33] The first bombardment took place on December 14, 1940, and the new element was first identified through oxidation on the night of February 23–24, 1941.[60]
A paper documenting the discovery was prepared by the team and sent to the journal Physical Review in March 1941,[33] but publication was delayed until a year after the end of World War II due to security concerns.[62] At the Cavendish Laboratory in Cambridge, Egon Bretscher and Norman Feather realized that a slow neutron reactor fuelled with uranium would theoretically produce substantial amounts of plutonium-239 as a by-product. They calculated that element 94 would be fissile, and had the added advantage of being chemically different from uranium, and could easily be separated from it.[21]
McMillan had recently named the first transuranic element neptunium after the planet Neptune, and suggested that element 94, being the next element in the series, be named for what was then considered the next planet, Pluto.[4][note 2] Nicholas Kemmer of the Cambridge team independently proposed the same name, based on the same reasoning as the Berkeley team.[21] Seaborg originally considered the name "plutium", but later thought that it did not sound as good as "plutonium".[64] He chose the letters "Pu" as a joke, in reference to the interjection "P U" to indicate an especially disgusting smell, which passed without notice into the periodic table.[note 3] Alternative names considered by Seaborg and others were "ultimium" or "extremium" because of the erroneous belief that they had found the last possible element on the periodic table.[66]
الأبحاث المبكرة
The chemistry of plutonium was found to resemble uranium after a few months of initial study.[33] Early research was continued at the secret Metallurgical Laboratory of the University of Chicago. On August 20, 1942, a trace quantity of this element was isolated and measured for the first time. About 50 micrograms of plutonium-239 combined with uranium and fission products was produced and only about 1 microgram was isolated.[44][67] This procedure enabled chemists to determine the new element's atomic weight.[68][note 4] On December 2, 1942, on a racket court under the west grandstand at the University of Chicago's Stagg Field, researchers headed by Enrico Fermi achieved the first self-sustaining chain reaction in a graphite and uranium pile known as CP-1. Using theoretical information garnered from the operation of CP-1, DuPont constructed an air-cooled experimental production reactor, known as X-10, and a pilot chemical separation facility at Oak Ridge. The separation facility, using methods developed by Glenn T. Seaborg and a team of researchers at the Met Lab, removed plutonium from uranium irradiated in the X-10 reactor. Information from CP-1 was also useful to Met Lab scientists designing the water-cooled plutonium production reactors for Hanford. Construction at the site began in mid-1943.[69]
In November 1943 some plutonium trifluoride was reduced to create the first sample of plutonium metal: a few micrograms of metallic beads.[44] Enough plutonium was produced to make it the first synthetically made element to be visible with the unaided eye.[70]
The nuclear properties of plutonium-239 were also studied; researchers found that when it is hit by a neutron it breaks apart (fissions) by releasing more neutrons and energy. These neutrons can hit other atoms of plutonium-239 and so on in an exponentially fast chain reaction. This can result in an explosion large enough to destroy a city if enough of the isotope is concentrated to form a critical mass.[33]
During the early stages of research, animals were used to study the effects of radioactive substances on health. These studies began in 1944 at the University of California at Berkeley's Radiation Laboratory and were conducted by Joseph G. Hamilton. Hamilton was looking to answer questions about how plutonium would vary in the body depending on exposure mode (oral ingestion, inhalation, absorption through skin), retention rates, and how plutonium would be fixed in tissues and distributed among the various organs. Hamilton started administering soluble microgram portions of plutonium-239 compounds to rats using different valence states and different methods of introducing the plutonium (oral, intravenous, etc.). Eventually, the lab at Chicago also conducted its own plutonium injection experiments using different animals such as mice, rabbits, fish, and even dogs. The results of the studies at Berkeley and Chicago showed that plutonium's physiological behavior differed significantly from that of radium. The most alarming result was that there was significant deposition of plutonium in the liver and in the "actively metabolizing" portion of bone. Furthermore, the rate of plutonium elimination in the excreta differed between species of animals by as much as a factor of five. Such variation made it extremely difficult to estimate what the rate would be for human beings.[71]
الانتاج أثناء مشروع منهاتن
During World War II the U.S. government established the Manhattan Project, which was tasked with developing an atomic bomb. The three primary research and production sites of the project were the plutonium production facility at what is now the Hanford Site, the uranium enrichment facilities at Oak Ridge, Tennessee, and the weapons research and design laboratory, now known as Los Alamos National Laboratory.[72]


The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the Oak Ridge National Laboratory.[33][note 5]
In January 1944, workers laid the foundations for the first chemical separation building, T Plant located in 200-West. Both the T Plant and its sister facility in 200-West, the U Plant, were completed by October. (U Plant was used only for training during the Manhattan Project.) The separation building in 200-East, B Plant, was completed in February 1945. The second facility planned for 200-East was canceled. Nicknamed Queen Marys by the workers who built them, the separation buildings were awesome canyon-like structures 800 feet long, 65 feet wide, and 80 feet high containing forty process pools. The interior had an eerie quality as operators behind seven feet of concrete shielding manipulated remote control equipment by looking through television monitors and periscopes from an upper gallery. Even with massive concrete lids on the process pools, precautions against radiation exposure were necessary and influenced all aspects of plant design.[69]
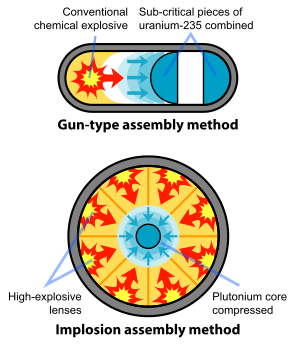
On April 5, 1944, Emilio Segrè at Los Alamos received the first sample of reactor-produced plutonium from Oak Ridge.[74] Within ten days, he discovered that reactor-bred plutonium had a higher concentration of the isotope plutonium-240 than cyclotron-produced plutonium. Plutonium-240 has a high spontaneous fission rate, raising the overall background neutron level of the plutonium sample.[75] The original gun-type plutonium weapon, code-named "Thin Man", had to be abandoned as a result—the increased number of spontaneous neutrons meant that nuclear pre-detonation (fizzle) was likely.[76]
The entire plutonium weapon design effort at Los Alamos was soon changed to the more complicated implosion device, code-named "Fat Man". With an implosion weapon, plutonium is compressed to a high density with explosive lenses—a technically more daunting task than the simple gun-type design, but necessary to use plutonium for weapons purposes. Enriched uranium, by contrast, can be used with either method.[76]
Construction of the Hanford B Reactor, the first industrial-sized nuclear reactor for the purposes of material production, was completed in March 1945. B Reactor produced the fissile material for the plutonium weapons used during World War II.[note 6] B, D and F were the initial reactors built at Hanford, and six additional plutonium-producing reactors were built later at the site.[79]
By the end of January 1945, the highly purified plutonium underwent further concentration in the completed chemical isolation building, where remaining impurities were removed successfully. Los Alamos received its first plutonium from Hanford on February 2. While it was still by no means clear that enough plutonium could be produced for use in bombs by the war's end, Hanford was by early 1945 in operation. Only two years had passed since Col. Franklin Matthias first set up his temporary headquarters on the banks of the Columbia River.[69]
According to Kate Brown, the plutonium production plants at Hanford and Mayak in Russia, over a period of four decades, "both released more than 200 million curies of radioactive isotopes into the surrounding environment — twice the amount expelled in the Chernobyl disaster in each instance".[80] Most of this radioactive contamination over the years were part of normal operations, but unforeseen accidents did occur and plant management kept this secret, as the pollution continued unabated.[80]
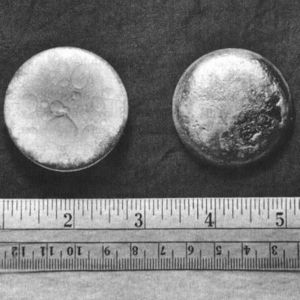
In 2004, a safe was discovered during excavations of a burial trench at the Hanford nuclear site. Inside the safe were various items, including a large glass bottle containing a whitish slurry which was subsequently identified as the oldest sample of weapons-grade plutonium known to exist. Isotope analysis by Pacific Northwest National Laboratory indicated that the plutonium in the bottle was manufactured in the X-10 Graphite Reactor at Oak Ridge during 1944.[81][82][83]
قنابل ترينيتي والرجل البدين الذرية
The first atomic bomb test, codenamed "Trinity" and detonated on July 16, 1945, near Alamogordo, New Mexico, used plutonium as its fissile material.[44] The implosion design of "the gadget", as the Trinity device was code-named, used conventional explosive lenses to compress a sphere of plutonium into a supercritical mass, which was simultaneously showered with neutrons from the "Urchin", an initiator made of polonium and beryllium (neutron source: (α, n) reaction).[33] Together, these ensured a runaway chain reaction and explosion. The overall weapon weighed over 4 tonnes, although it used just 6.2 kg of plutonium in its core.[84] About 20% of the plutonium used in the Trinity weapon underwent fission, resulting in an explosion with an energy equivalent to approximately 20,000 tons of TNT.[85][note 7]
An identical design was used in the "Fat Man" atomic bomb dropped on Nagasaki, Japan, on August 9, 1945, killing 35,000–40,000 people and destroying 68%–80% of war production at Nagasaki.[87] Only after the announcement of the first atomic bombs was the existence and name of plutonium made known to the public by the Manhattan Project's Smyth Report.[88]
الاستخدام والفاقد في الحرب الباردة
Large stockpiles of weapons-grade plutonium were built up by both the Soviet Union and the United States during the Cold War. The U.S. reactors at Hanford and the Savannah River Site in South Carolina produced 103 tonnes,[89] and an estimated 170 tonnes of military-grade plutonium was produced in the USSR.[90][note 8] Each year about 20 tonnes of the element is still produced as a by-product of the nuclear power industry.[10] As much as 1000 tonnes of plutonium may be in storage with more than 200 tonnes of that either inside or extracted from nuclear weapons.[33] SIPRI estimated the world plutonium stockpile in 2007 as about 500 tonnes, divided equally between weapon and civilian stocks.[92]
Radioactive contamination at the Rocky Flats Plant primarily resulted from two major plutonium fires in 1957 and 1969. Much lower concentrations of radioactive isotopes were released throughout the operational life of the plant from 1952 to 1992. Prevailing winds from the plant carried airborne contamination south and east, into populated areas northwest of Denver. The contamination of the Denver area by plutonium from the fires and other sources was not publicly reported until the 1970s. According to a 1972 study coauthored by Edward Martell, "In the more densely populated areas of Denver, the Pu contamination level in surface soils is several times fallout", and the plutonium contamination "just east of the Rocky Flats plant ranges up to hundreds of times that from nuclear tests".[93] As noted by Carl Johnson in Ambio, "Exposures of a large population in the Denver area to plutonium and other radionuclides in the exhaust plumes from the plant date back to 1953."[94] Weapons production at the Rocky Flats plant was halted after a combined FBI and EPA raid in 1989 and years of protests. The plant has since been shut down, with its buildings demolished and completely removed from the site.[95]
In the U.S., some plutonium extracted from dismantled nuclear weapons is melted to form glass logs of plutonium oxide that weigh two tonnes.[33] The glass is made of borosilicates mixed with cadmium and gadolinium.[note 9] These logs are planned to be encased in stainless steel and stored as much as 4 km (2 mi) underground in bore holes that will be back-filled with concrete.[33] The U.S. planned to store plutonium in this way at the Yucca Mountain nuclear waste repository, which is about 100 ميل (160 km) north-east of Las Vegas, Nevada.[96]
On March 5, 2009, Energy Secretary Steven Chu told a Senate hearing "the Yucca Mountain site no longer was viewed as an option for storing reactor waste".[97] Starting in 1999, military-generated nuclear waste is being entombed at the Waste Isolation Pilot Plant in New Mexico.
In a Presidential Memorandum dated January 29, 2010, President Obama established the Blue Ribbon Commission on America's Nuclear Future.[98] In their final report the Commission put forth recommendations for developing a comprehensive strategy to pursue, including:[99]
- "Recommendation #1: The United States should undertake an integrated nuclear waste management program that leads to the timely development of one or more permanent deep geological facilities for the safe disposal of spent fuel and high-level nuclear waste".[99]
التجريب الطبي
During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects.[100] Animal studies found that a few milligrams of plutonium per kilogram of tissue is a lethal dose.[101]
In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition.[100] This was reduced to one microgram in July 1945 after animal studies found that the way plutonium distributed itself in bones was more dangerous than radium.[101] Most of the subjects, Eileen Welsome says, were poor, powerless, and sick.[102]
From 1945 to 1947, eighteen human test subjects were injected with plutonium without informed consent. The tests were used to create diagnostic tools to determine the uptake of plutonium in the body in order to develop safety standards for working with plutonium.[100] Ebb Cade was an unwilling participant in medical experiments that involved injection of 4.7 micrograms of Plutonium on 10 April 1945 at Oak Ridge, Tennessee.[103][104] This experiment was under the supervision of Harold Hodge.[105] Other experiments directed by the United States Atomic Energy Commission and the Manhattan Project continued into the 1970s. The Plutonium Files chronicles the lives of the subjects of the secret program by naming each person involved and discussing the ethical and medical research conducted in secret by the scientists and doctors. The episode is now considered to be a serious breach of medical ethics and of the Hippocratic Oath.[106]
The government covered up most of these radiation mishaps until 1993, when President Bill Clinton ordered a change of policy and federal agencies then made available relevant records. The resulting investigation was undertaken by the president's Advisory Committee on Human Radiation Experiments, and it uncovered much of the material about plutonium research on humans. The committee issued a controversial 1995 report which said that "wrongs were committed" but it did not condemn those who perpetrated them.[102]
الاستخدامات
النظير 239Pu is a key fissile component in nuclear weapons, due to its ease of fissioning and availability. The critical mass for an unreflected sphere of plutonium is 16 kg, but through the use of a neutron-reflecting tamper the pit of plutonium in a fission bomb is reduced to 10 kg, which is a sphere with a diameter of 10 cm. The Manhattan Project "Fat Man" type plutonium bombs, using explosive compression of Pu to significantly higher densities than normal, were able to function with plutonium cores of only 6.2 kg.[107] Complete detonation may be achieved through the use of an additional neutron source (often from a small amount of fusion fuel). The Fat Man bomb had an explosive yield of 21 kilotons. (See also nuclear weapon design.)
The isotope plutonium-238 (238Pu) has a half-life of 88 years and emits a large amount of thermal energy as it decays. Being an alpha emitter, it combines high energy radiation with low penetration (thereby requiring minimal shielding). These characteristics make it well suited for electrical power generation for devices which must function without direct maintenance for timescales approximating a human lifetime. It is therefore used in radioisotope thermoelectric generators such as those powering the Cassini and New Horizons (Pluto) space probes; earlier versions of the same technology powered the ALSEP and EASEP systems including seismic experiments on the Apollo Moon missions.
238Pu has been used successfully to power artificial heart pacemakers, to reduce the risk of repeated surgery.[بحاجة لمصدر] It has been largely replaced by lithium-based primary cells, but as of 2003 there were somewhere between 50 and 100 plutonium-powered pacemakers still implanted and functioning in living patients.
التواجد
While almost all plutonium is manufactured synthetically, extremely tiny trace amounts are found naturally in uranium ores. These come about by a process of neutron capture by 238U nuclei, initially forming 239U; two subsequent beta decays then form 239Pu (with a 239Np intermediary), which has a half-life of 24,110 years. This is also the process used to manufacture 239Pu in nuclear reactors. Some traces of 244Pu remain[بحاجة لمصدر] from the birth of the solar system from the waste of supernovae, because its half-life of 80 million years is fairly long.
A relatively high concentration of plutonium was discovered at the natural nuclear fission reactor in Oklo, Gabon in 1972. Since 1945, approximately 7700 kg has been released onto Earth through nuclear explosions.
التصنيع
Pu-240, Pu-241 و Pu-242
The activation cross section for 239Pu is 270 barns, while the fission cross section is 747 barns for thermal neutrons. The higher plutonium isotopes are created when the uranium fuel is used for a long time. It is the case that for high burnup used fuel that the concentrations of the higher plutonium isotopes will be higher than the low burnup fuel which is reprocessed to obtain bomb grade plutonium.
| العنصر | النظير | مقطع عرضي لنيوترون حراري |
decay mode | نصف العمر |
|---|---|---|---|---|
| U | 238 | 2.7 | α | 4.47 x 109 سنة |
| U | 239 | - | β | 23 دقيقة |
| Np | 239 | - | β | 2.36 يوم |
| Pu | 239 | 270 (capture) | α | 24110 سنة |
| Pu | 240 | 289 (capture) | α | 6564 سنة |
| Pu | 241 | 362 (capture) | β | 14.35 سنة |
| Pu | 242 | 18.8 | α | 373300 سنة |
Pu-239
 مقالة مفصلة: پلوتونيوم-239
مقالة مفصلة: پلوتونيوم-239
پلوتونيوم-239 is one of the three fissile materials used for the production of nuclear weapons and in some nuclear reactors as a source of energy. The other fissile materials are uranium-235 and uranium-233. Plutonium-239 is virtually nonexistent in nature. It is made by bombarding uranium-238 with neutrons in a nuclear reactor. Uranium-238 is present in quantity in most reactor fuel; hence plutonium-239 is continuously made in these reactors. Since plutonium-239 can itself be split by neutrons to release energy, plutonium-239 provides a portion of the energy generation in a nuclear reactor.
| العنصر | النظير | Thermal neutron cross section |
decay mode | نصف العمر |
|---|---|---|---|---|
| U | 238 | 2.7 | α | 4.47 x 109 سنة |
| U | 239 | - | β | 23 دقيقة |
| Np | 239 | - | β | 2.36 يوم |
| Pu | 239 | - | α | 24110 سنة |
Pu-238
 مقالة مفصلة: پلوتونيوم-238
مقالة مفصلة: پلوتونيوم-238
There are small amounts of Pu-238 in the plutonium of usual plutonium-producing reactors. However, isotopic separation would be quite expensive compared to another method: when a U-235 atom captures a neutron, it is converted to an excited state of U-236. Some of the excited U-236 nuclei undergo fission, but some decay to the ground state of U-236 by emitting gamma radiation. Further neutron capture creates U-237 which has a half-life of 7 days and thus quickly decays to Np-237. Since nearly all neptunium is produced in this way or consists of isotopes which decay quickly, one gets nearly pure Np-237 by chemical separation of neptunium. After this chemical separation, Np-237 is again irradiated by reactor neutrons to be converted to Np-238 which decays to Pu-238 with a half-life of 2 days.
| العنصر | النظير | مقطع عرضي لنيوترون حراري |
decay mode | نصف العمر |
|---|---|---|---|---|
| U | 235 | 99 | α | 703800000 years |
| U | 236 | 5.3 | α | 23420000 years |
| U | 237 | - | β | 6.75 days |
| Np | 237 | 165 (capture) | α | 2144000 years |
| Np | 238 | - | β | 2.11 days |
| Pu | 238 | - | α | 87.7 years |
المركبات
ويتفاعل الپلوتونيوم بسهولة مع الاكسجين، مكوناً PuO وPuO2، وأكاسيد وسيطة. ويتفاعل مع الهالوجينات، منتجاً مركبات مثل PuX3 حيث X يمكن أن تكون F، Cl، Br أو I; PuF4 و PuF6 يُشاهـَدوا. ويُشاهـَد الأكسيهاليدات التالية: PuOCl, PuOBr و PuOI. وسيتفاعل مع الكربون ليشكل PuC، والنيتروجين ليشكل PuN والسليكون ليشكل PuSi2.
الپلوتونيوم ومثل الأكتينيدات الأخرى فإنه يشكـِّل بسهولة قلب من ثاني أكسيد الپلوتونيل (PuO2). وفي البيئة، هذا القلب من البلوتونيل يتعقد بتلقائية مع الكربونات وكذلك باقي moieties الأكسجين (OH-, NO2-, NO3-, and SO4-2) ليشكل معقدات مشحونة التي يمكن أن تكون قابلة تلقائياً للحركة with low affinities to soil.
- PuO2(CO3)1-2
- PuO2(CO3)2-4
- PuO2(CO3)3-6
PuO2 formed from neutralizing highly acidic nitric acid solutions tends to form polymeric PuO2 which is resistant to complexation. Plutonium also readily shifts valences between the +3, +4, +5 and +6 states. It is common for some fraction of plutonium in solution to exist in all of these states in equilibrium.
يشكل البلوتونيوم مع الأكسجين ثنائي الأكسيد PuO2 الذي يُعد من أكثر مركبات البلوتونيوم أهمية، وإن درجة انصهاره العالية البالغة نحو 2400ْس، وثباته الكيمياوي والإشعاعي، وتشابهه مع ثنائي أكسيد اليورانيوم UO2 تجعل منه وقوداً ممتازاً للمفاعلات النووية. ويحضر PuO2 بتسخين البلوتونيوم أو أيٍ من مركباته، عدا الفسفات في أكسجين الهواء، في درجة تراوح بين 870ْس و1200ْس. ويشكل مع الهالوجينات عدداً من الهاليدات: ثلاثي الهاليد PuX3، (F=X، Br Cl، I)، ورباعي الفلوريد PuF4 وسداسي الفلوريد PuF6.
ويشكل البلوتونيوم حماضات ثابتة بحالات الأكسدة III وIV وVI. وتُعرف مركبات كثيرة أخرى للبلوتونيوم من بينها: كربيدات البلوتونيوم PuC وPu2C3، سيليسيدات البلوتونيوم α-PuSi2 وß-PuSi2 وكبريتيدات البلوتونيوم PuS، Pu2S3 وPu3S4 ونتريد البلوتونيوم PuN.
التآصلات
 مقالة مفصلة: تآصلات الپلوتونيوم
مقالة مفصلة: تآصلات الپلوتونيوم
حتى عند الضغوط العادية، يتواجد الپلوتونيوم في العديد من التآصلات. These allotropes differ widely in crystal structure and density; the α and δ allotropes differ in density by more than 25% at constant pressure.
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. The reasons for the complicated phase diagram are not entirely understood; recent research has focused on constructing accurate computer models of the phase transitions.
In weapons applications, plutonium is often alloyed with another metal (e.g., delta phase with a small percentage of گاليوم) to increase phase stability and thereby enhance workability and ease of handling. Interestingly, in fission weapons, the explosive shock waves used to compress a plutonium core will also cause a transition from the usual delta phase plutonium to the denser alpha phase, significantly helping to achieve supercriticality.
النظائر
 مقالة مفصلة: نظائر الپلوتونيوم
مقالة مفصلة: نظائر الپلوتونيوم
Twenty-one plutonium radioisotopes have been characterized. The most stable are Pu-244, with a half-life of 80.8 million years, Pu-242, with a half-life of 373,300 years, and Pu-239, with a half-life of 24,110 years. Because of its comparatively large half-life, minute amounts of Pu-244 can be found in nature[108], All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states, though none are very stable (all have half-lives less than one second).
The isotopes of plutonium range in atomic weight from 228.0387 u (Pu-228) to 247.074 u (Pu-247). The primary decay modes before the most stable isotope, Pu-244, are spontaneous fission and alpha emission; the primary mode after is beta emission. The primary decay products before Pu-244 are uranium and neptunium isotopes (neglecting the wide range of daughter nuclei created by fission processes), and the primary products after are americium isotopes.

Key isotopes for applications are Pu-239, which is suitable for use in nuclear weapons and nuclear reactors, and Pu-238, which is suitable for use in radioisotope thermoelectric generators; see above for more details. The isotope Pu-240 undergoes spontaneous fission very readily, and is produced when Pu-239 is exposed to neutrons. The presence of Pu-240 in a material limits its nuclear bomb potential since it emits neutrons randomly, increasing the difficulty of initiating accurately the chain reaction at the desired instant and thus reducing the bomb's reliability and power. Plutonium consisting of more than about 90% Pu-239 is called weapons-grade plutonium; plutonium obtained from commercial reactors generally contains at least 20% Pu-240 and is called reactor-grade plutonium.
Pu-240, while of little importance by itself, plays a crucial role as a contaminant in plutonium used in nuclear weapons. It spontaneously fissions at a high rate, and a 1% impurity in Pu-239 will lead to unacceptably early initiation of a fission chain reaction in gun-type atomic weapons (e.g. the proposed Thin Man bomb), blowing the weapon apart before much of its material can fission. Pu-240 contamination is the reason plutonium weapons must use an implosion design. A theoretical 100% pure Pu-239 weapon could be constructed as a gun-type device, but achieving this level of purity is prohibitively difficult. Pu-240 contamination has proven a mixed blessing to weapons designers. While it created delays and headaches during the Manhattan Project because of the need to develop implosion technology, those very same difficulties are currently a barrier to nuclear proliferation. Implosion devices are also inherently more efficient and less prone toward accidental detonation than are gun-type weapons.
محاذير
تكمن المخاطر الأساسية للبلوتونيوم في نشاطه الإشعاعي وطاقته النووية وفعاليته الكيماوية كمعدن. وإن التعامل مع البلوتونيوم الذي يشع جسيمات ألفا (α) لا يشكل صعوبة، إذ لا تحتاج الوقاية منها إلى ملابس خاصة، ولكن المشكلة تنشأ عند التعامل مع النظائر الأخرى (غير 239 Pu) التي تصدر أشعة غاما الضعيفة. ويعزى خطر البلوتونيوم الشديد على الصحة إلى توضعه في نقي العظام حيث تتشكل الكريات الدموية. ويستطيع البلوتونيوم دخول الجسم عن طريق الجروح والجلد وجهازي الهضم والتنفس، ويعد دخوله عن طريق التنفس أكثر الطرق احتمالاً، لذا يجري التعامل معه بوساطة تجهيزات خاصة، ضغطها يقل بنحو 0.001 جو عن الضغط الجوي المحيط. إن أفضل التقنيات المستخدمة في إخماد حرائق البلوتونيوم تعتمد على عزل الأكسجين عنه، وذلك إما بجعل الجو المحيط خاملاً وإما بالرش بمسحوق الگرافيت أو أكسيد المغنسيوم.
السمية
كل نظائر ومركبات الپلوتونيوم سامة ومشعة. While plutonium is sometimes described in media reports as "the most toxic substance known to man", from the standpoint of actual chemical or radiological toxicity this is incorrect. When taken in by mouth, plutonium is less poisonous than if inhaled, since it is not absorbed into the body efficiently when ingested. The U.S. Department of Energy estimates the increase in lifetime cancer risk for inhaled plutonium as 3×10−8 pCi−1.[109] (this means that inhaling 1 μCi, or about 2.5 μg of reactor-grade plutonium is estimated to increase one's lifetime risk of developing cancer as a result of the exposure to 3%). When plutonium is absorbed into the body, it is excreted very slowly, with a biological half-life of 200 years.[110] From a purely chemical standpoint, it is about as poisonous as lead and other heavy metals. [بحاجة لمصدر] Not surprisingly, it has a metallic taste.[111]
Plutonium may be extremely dangerous when handled incorrectly. The alpha radiation it emits does not penetrate the skin, but can irradiate internal organs when plutonium is inhaled or ingested. Particularly at risk are the skeleton, where it is likely to be absorbed by the bone surface, and the liver, where it will likely collect and become concentrated. Approximately 0.008 microcuries absorbed in bone marrow is the maximum withstandable dose. Anything more is considered toxic. Extremely fine particles of plutonium (on the order of micrograms) can cause lung cancer if inhaled.[بحاجة لمصدر]
Other substances including ricin, tetrodotoxin, botulinum toxin, and tetanus toxin are fatal in doses of (sometimes far) under one milligram, and others (the nerve agents, the amanita toxin) are in the range of a few milligrams. As such, plutonium is not unusual in terms of toxicity, even by inhalation. In addition, those substances are fatal in hours to days, whereas plutonium (and other cancer-causing radioactives) give an increased chance of illness decades in the future. Considerably larger amounts may cause acute radiation poisoning and death if ingested or inhaled; however, so far, no human is known to have immediately died because of inhaling or ingesting plutonium and many people have measurable amounts of plutonium in their bodies.[بحاجة لمصدر]
مشاكل التخلص منه
In contrast to naturally occurring radioisotopes such as radium or C-14, plutonium was manufactured, concentrated, and isolated in large amounts (hundreds of metric tons) during the Cold War for weapons production. These stockpiles, whether or not in weapons form, pose a significant problem because, unlike chemical or biological agents, no chemical process can destroy them. One proposal to dispose of surplus weapons-grade plutonium is to mix it with highly radioactive isotopes (e.g., spent reactor fuel) to deter handling by potential thieves or terrorists. Another is to mix it with uranium and use it to fuel nuclear power reactors (the mixed oxide or MOX approach). This would not only fission (and thereby destroy) much of the Pu-239, but also transmute a significant fraction of the remainder into Pu-240 and heavier isotopes that would make the resulting mixture useless for nuclear weapons.[112]
Criticality potential
Toxicity issues aside, care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235's. Despite not being confined by external pressure as is required for a nuclear weapon, it will nevertheless heat itself and break whatever confining environment it is in. Shape is relevant; compact shapes such as spheres are to be avoided. Plutonium in solution is more likely to form a critical mass than the solid form (due to moderation by the hydrogen in water). A weapon-scale nuclear explosion cannot occur accidentally, since it requires a greatly supercritical mass in order to explode rather than simply melt or fragment. However, a marginally critical mass will cause a lethal dose of radiation and has in fact done so in the past on several occasions.
Criticality accidents have occurred in the past, some of them with lethal consequences. Careless handling of tungsten carbide bricks around a 6.2 kg plutonium sphere resulted in a lethal dose of radiation at Los Alamos on August 21, 1945, when scientist Harry K. Daghlian, Jr. received a dose estimated to be 510 rems (5.1 Sv) and died four weeks later. Nine months later, another Los Alamos scientist, Louis Slotin, died from a similar accident involving a beryllium reflector and the same plutonium core (the so-called "demon core") that had previously claimed the life of Daghlian. These incidents were fictionalized in the 1989 film Fat Man and Little Boy. In 1958, during a process of purifying plutonium at Los Alamos, a critical mass was formed in a mixing vessel, which resulted in the death of a crane operator. Other accidents of this sort have occurred in the الاتحاد السوڤيتي, Japan, and many other countries. (See List of nuclear accidents.) The 1986 Chernobyl accident caused a minor release of plutonium.[بحاجة لمصدر]
قابلية الاشتعال
Metallic plutonium is also a fire hazard, especially if the material is finely divided. It reacts chemically with oxygen and water, which may result in an accumulation of plutonium hydride, a pyrophoric substance; that is, a material that will ignite in air at room temperature. Plutonium expands considerably in size as it oxidizes and thus may break its container. The radioactivity of the burning material is an additional hazard. Magnesium-oxide sand is the most effective material for extinguishing a plutonium fire. It cools the burning material, acting as a heat sink, and also blocks off oxygen. There was a major plutonium-initiated fire at the Rocky Flats Plant near Boulder, كولورادو in 1969.[113] To avoid these problems, special precautions are necessary to store or handle plutonium in any form; generally a dry inert atmosphere is required.[114]
انظر أيضاً
المضادر
- ^ أ ب ت ث "Plutonium, Radioactive". Wireless Information System for Emergency Responders (WISER). Bethesda (MD): U.S. National Library of Medicine, National Institutes of Health. Archived from the original on August 22, 2011. Retrieved November 23, 2008. (public domain text)
- ^
"Nitric acid processing". Actinide Research Quarterly. Los Alamos (NM): Los Alamos National Laboratory (3rd quarter). 2008. Retrieved February 9, 2010.
While plutonium dioxide is normally olive green, samples can be various colors. It is generally believed that the color is a function of chemical purity, stoichiometry, particle size, and method of preparation, although the color resulting from a given preparation method is not always reproducible.
- ^ أ ب ت Sonzogni, Alejandro A. (2008). "Chart of Nuclides". Upton: National Nuclear Data Center, Brookhaven National Laboratory. Retrieved September 13, 2008.
- ^ أ ب ت ث ج Heiserman 1992, p. 338
- ^ Rhodes 1986, pp. 659–660 Leona Marshall: "When you hold a lump of it in your hand, it feels warm, like a live rabbit"
- ^ أ ب ت ث Miner 1968, p. 544
- ^ أ ب ت ث ج ح خ Hecker, Siegfried S. (2000). "Plutonium and its alloys: from atoms to microstructure" (PDF). Los Alamos Science. 26: 290–335. Retrieved February 15, 2009.
- ^ Hecker, Siegfried S.; Martz, Joseph C. (2000). "Aging of Plutonium and Its Alloys" (PDF). Los Alamos Science. Los Alamos, New Mexico: Los Alamos National Laboratory (26): 242. Retrieved February 15, 2009.
- ^ أ ب ت ث Baker, Richard D.; Hecker, Siegfried S.; Harbur, Delbert R. (1983). "Plutonium: A Wartime Nightmare but a Metallurgist's Dream" (PDF). Los Alamos Science. Los Alamos National Laboratory: 148, 150–151. Retrieved February 15, 2009.
- ^ أ ب ت ث Lide 2006, pp. 4–27
- ^ أ ب ت ث Miner 1968, p. 542
- ^ "Plutonium Crystal Phase Transitions". GlobalSecurity.org.
- ^ "Glossary – Fissile material". United States Nuclear Regulatory Commission. November 20, 2014. Retrieved February 5, 2015.
- ^ Asimov 1988, p. 905
- ^ Glasstone, Samuel; Redman, Leslie M. (June 1972). "An Introduction to Nuclear Weapons" (PDF). Atomic Energy Commission Division of Military Applications. p. 12. WASH-1038. Archived from the original (PDF) on August 27, 2009.
- ^ Gosling 1999, p. 40
- ^ "Plutonium: The First 50 Years" (PDF). U.S. Department of Energy. 1996. DOE/DP-1037. Archived from the original (PDF) on February 18, 2013.
- ^ Heiserman 1992, p. 340
- ^ Kennedy, J. W.; Seaborg, G. T.; Segrè, E.; Wahl, A. C. (1946). "Properties of Element 94". Physical Review. 70 (7–8): 555–556. Bibcode:1946PhRv...70..555K. doi:10.1103/PhysRev.70.555.
- ^ Greenwood 1997, p. 1259
- ^ أ ب ت Clark 1961, pp. 124–125.
- ^ Seaborg, Glenn T.; McMillan, E.; Kennedy, J. W.; Wahl, A. C. (1946). "Radioactive Element 94 from Deuterons on Uranium". Physical Review. 69 (7–8): 366. Bibcode:1946PhRv...69..366S. doi:10.1103/PhysRev.69.366.
- ^ Bernstein 2007, pp. 76–77.
- ^ "Can Reactor Grade Plutonium Produce Nuclear Fission Weapons?". Council for Nuclear Fuel Cycle Institute for Energy Economics, Japan. May 2001.
- ^ Heiserman 1992, p. 339
- ^ Crooks, William J. (2002). "Nuclear Criticality Safety Engineering Training Module 10 – Criticality Safety in Material Processing Operations, Part 1" (PDF). Archived from the original (PDF) on March 20, 2006. Retrieved February 15, 2006.
- ^ Matlack, George (2002). A Plutonium Primer: An Introduction to Plutonium Chemistry and its Radioactivity. Los Alamos National Laboratory. LA-UR-02-6594.
- ^ Windorff, Cory J.; Chen, Guo P; Cross, Justin N; Evans, William J.; Furche, Filipp; Gaunt, Andrew J.; Janicke, Michael T.; Kozimor, Stosh A.; Scott, Brian L. (2017). "Identification of the Formal +2 Oxidation State of Plutonium: Synthesis and Characterization of {PuII[C5H3(SiMe3)2]3}−". J. Am. Chem. Soc. 139 (11): 3970–3973. doi:10.1021/jacs.7b00706. PMID 28235179.
- ^ أ ب Zaitsevskii, Andréi; Mosyagin, Nikolai S.; Titov, Anatoly V.; Kiselev, Yuri M. (21 July 2013). "Relativistic density functional theory modeling of plutonium and americium higher oxide molecules". The Journal of Chemical Physics. 139 (3): 034307. doi:10.1063/1.4813284.
- ^ Kiselev, Yu. M.; Nikonov, M. V.; Dolzhenko, V. D.; Ermilov, A. Yu.; Tananaev, I. G.; Myasoedov, B. F. (17 January 2014). "On existence and properties of plutonium(VIII) derivatives". Radiochimica Acta. 102 (3). doi:10.1515/ract-2014-2146.
- ^ Eagleson 1994, p. 840
- ^ أ ب ت ث Miner 1968, p. 545
- ^ أ ب ت ث ج ح خ د ذ ر ز س Emsley 2001, pp. 324–329
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةNucSafety - ^ Crooks, W. J.; et al. (2002). "Low Temperature Reaction of ReillexTM HPQ and Nitric Acid". Solvent Extraction and Ion Exchange. 20 (4–5): 543–559. doi:10.1081/SEI-120014371.
- ^ أ ب Dumé, Belle (November 20, 2002). "Plutonium is also a superconductor". PhysicsWeb.org.
- ^ Moody, Hutcheon & Grant 2005, p. 169
- ^ Kolman, D. G. & Colletti, L. P. (2009). "The aqueous corrosion behavior of plutonium metal and plutonium–gallium alloys exposed to aqueous nitrate and chloride solutions". ECS Transactions. Electrochemical Society. 16 (52): 71. Bibcode:2009ECSTr..16Z..71K. doi:10.1149/1.3229956. ISBN 978-1-56677-751-3.
- ^ Hurst & Ward 1956
- ^ Curro, N. J. (Spring 2006). "Unconventional superconductivity in PuCoGa5" (PDF). Los Alamos National Laboratory. Archived from the original (PDF) on July 22, 2011. Retrieved January 24, 2010.
- ^ McCuaig, Franklin D. "Pu–Zr alloy for high-temperature foil-type fuel" U.S. Patent 4٬059٬439, Issued on November 22, 1977
- ^ Jha 2004, p. 73
- ^ أ ب ت Kay 1965, p. 456
- ^ أ ب ت ث Miner 1968, p. 541
- ^ "Oklo: Natural Nuclear Reactors". U.S. Department of Energy, Office of Civilian Radioactive Waste Management. 2004. Archived from the original on October 20, 2008. Retrieved November 16, 2008.
- ^ Curtis, David; Fabryka-Martin, June; Paul, Dixon; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275–285. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- ^ Bernstein 2007, pp. 75–77.
- ^ Hoffman, D. C.; Lawrence, F. O.; Mewherter, J. L.; Rourke, F. M. (1971). "Detection of Plutonium-244 in Nature". Nature. 234 (5325): 132–134. Bibcode:1971Natur.234..132H. doi:10.1038/234132a0.
- ^ Peterson, Ivars (December 7, 1991). "Uranium displays rare type of radioactivity". Science News. Wiley-Blackwell. 140 (23): 373. doi:10.2307/3976137. JSTOR 3976137.
- ^ Hoffman, D. C.; Lawrence, F. O.; Mewherter, J. L.; Rourke, F. M. (1971). "Detection of Plutonium-244 in Nature". Nature. 234 (5325): 132–134. Bibcode:1971Natur.234..132H. doi:10.1038/234132a0. Nr. 34.
- ^ Turner, Grenville; Harrison, T. Mark; Holland, Greg; Mojzsis, Stephen J.; Gilmour, Jamie (2004-01-01). "Extinct 244Pu in Ancient Zircons" (PDF). Science. 306 (5693): 89–91. Bibcode:2004Sci...306...89T. doi:10.1126/science.1101014. JSTOR 3839259. PMID 15459384.
- ^ Hutcheon, I. D.; Price, P. B. (1972-01-01). "Plutonium-244 Fission Tracks: Evidence in a Lunar Rock 3.95 Billion Years Old". Science. 176 (4037): 909–911. Bibcode:1972Sci...176..909H. doi:10.1126/science.176.4037.909. JSTOR 1733798. PMID 17829301.
- ^ Kunz, Joachim; Staudacher, Thomas; Allègre, Claude J. (1998-01-01). "Plutonium-Fission Xenon Found in Earth's Mantle". Science. 280 (5365): 877–880. Bibcode:1998Sci...280..877K. doi:10.1126/science.280.5365.877. JSTOR 2896480.
- ^ Wallner, A.; Faestermann, T.; Feige, J.; Feldstein, C.; Knie, K.; Korschinek, G.; Kutschera, W.; Ofan, A.; Paul, M.; Quinto, F.; Rugel, G.; Steiner, P. (30 March 2014). "Abundance of live 244Pu in deep-sea reservoirs on Earth points to rarity of actinide nucleosynthesis". Nature Communications. 6: 5956. arXiv:1509.08054. Bibcode:2015NatCo...6E5956W. doi:10.1038/ncomms6956.
- ^ Holden, Norman E. (2001). "A Short History of Nuclear Data and Its Evaluation". 51st Meeting of the USDOE Cross Section Evaluation Working Group. Upton (NY): National Nuclear Data Center, Brookhaven National Laboratory. Retrieved January 3, 2009.
- ^ Fermi, Enrico (December 12, 1938). "Artificial radioactivity produced by neutron bombardment: Nobel Lecture" (PDF). Royal Swedish Academy of Sciences.
- ^ Darden, Lindley (1998). "The Nature of Scientific Inquiry". College Park: Department of Philosophy, University of Maryland. Retrieved January 3, 2008.
- ^ Bernstein 2007, pp. 44–52.
- ^ Seaborg, Glenn T. "An Early History of LBNL: Elements 93 and 94". Advanced Computing for Science Department, Lawrence Berkeley National Laboratory. Retrieved September 17, 2008.
- ^ أ ب Glenn T. Seaborg. "The plutonium story". Lawrence Berkeley Laboratory, University of California. LBL-13492, DE82 004551.
- ^ E. Segrè, A Mind Always in Motion, University of California Press, 1993, pp 162-169
- ^ Seaborg & Seaborg 2001, pp. 71–72.
- ^ Heiserman 1992, p. 338.
- ^ Clark, David L.; Hobart, David E. (2000). "Reflections on the Legacy of a Legend: Glenn T. Seaborg, 1912–1999" (PDF). Los Alamos Science. 26: 56–61, on 57. Retrieved February 15, 2009.
- ^ Clark, David L.; Hobart, David E. (2000). "Reflections on the Legacy of a Legend: Glenn T. Seaborg, 1912–1999" (PDF). Los Alamos Science. 26: 56–61, on 57. Retrieved February 15, 2009.
- ^ "Frontline interview with Seaborg". Frontline. Public Broadcasting Service. 1997. Retrieved December 7, 2008.
- ^
Glenn T. Seaborg (1977). "History of MET Lab Section C-I, April 1942 – April 1943". Office of Scientific & Technical Information Technical Reports (California Univ., Berkeley (USA). Lawrence Berkeley Lab.). doi:. Archived from the original. You must specify the date the archive was made using the
|archivedate=parameter. https://digital.library.unt.edu/ark:/67531/metadc1414500/. - ^ "Room 405, George Herbert Jones Laboratory". National Park Service. Archived from the original on February 8, 2008. Retrieved December 14, 2008.
- ^ أ ب ت "Periodic Table of Elements". Los Alamos National Laboratory. Retrieved September 15, 2015.
- ^ Miner 1968, p. 540
- ^ "Plutonium". Atomic Heritage Foundation. Retrieved September 15, 2015.
- ^ "Site Selection". LANL History. Los Alamos, New Mexico: Los Alamos National Laboratory. Retrieved December 23, 2008.
- ^ Hammel, E. F. (2000). "The taming of "49" – Big Science in little time. Recollections of Edward F. Hammel, In: Cooper N.G. Ed. Challenges in Plutonium Science" (PDF). Los Alamos Science. 26 (1): 2–9. Retrieved February 15, 2009.
- Hecker, S. S. (2000). "Plutonium: an historical overview. In: Challenges in Plutonium Science". Los Alamos Science. 26 (1): 1–2. Retrieved February 15, 2009.
- ^ Sublette, Carey. "Atomic History Timeline 1942–1944". Washington (DC): Atomic Heritage Foundation. Retrieved December 22, 2008.
- ^ Hoddeson et al. 1993, pp. 235–239.
- ^ أ ب Hoddeson et al. 1993, pp. 240–242.
- ^ Wahlen 1989, p. 1.
- ^ "Weekly List Actions". National Park Service. August 29, 2008. Retrieved August 30, 2008.
- ^ Wahlen 1989, p. iv, 1
- ^ أ ب Lindley, Robert (2013). "Kate Brown: Nuclear "Plutopias" the Largest Welfare Program in American History". History News Network.
- ^ Rincon, Paul (March 2, 2009). "BBC NEWS – Science & Environment – US nuclear relic found in bottle". BBC News. Retrieved March 2, 2009.
- ^ Gebel, Erika (2009). "Old plutonium, new tricks". Analytical Chemistry. 81 (5): 1724. doi:10.1021/ac900093b.
- ^ Schwantes, Jon M.; Matthew Douglas; Steven E. Bonde; James D. Briggs; et al. (2009). "Nuclear archeology in a bottle: Evidence of pre-Trinity U.S. weapons activities from a waste burial site". Analytical Chemistry. 81 (4): 1297–1306. doi:10.1021/ac802286a. PMID 19152306.
- ^ Sublette, Carey (July 3, 2007). "8.1.1 The Design of Gadget, Fat Man, and "Joe 1" (RDS-1)". Nuclear Weapons Frequently Asked Questions, edition 2.18. The Nuclear Weapon Archive. Retrieved January 4, 2008.
- ^ Malik, John (September 1985). "The Yields of the Hiroshima and Nagasaki Explosions" (PDF). Los Alamos. p. Table VI. LA-8819. Retrieved February 15, 2009.
- ^ On the figure of 1 kg = 17 kt, see Garwin, Richard (October 4, 2002). "Proliferation of Nuclear Weapons and Materials to State and Non-State Actors: What It Means for the Future of Nuclear Power" (PDF). University of Michigan Symposium. Federation of American Scientists. Retrieved January 4, 2009.
- ^ Sklar 1984, pp. 22–29.
- ^ Bernstein 2007, p. 70.
- ^ "Historic American Engineering Record: B Reactor (105-B Building)". Richland: U.S. Department of Energy. 2001. p. 110. DOE/RL-2001-16. Retrieved December 24, 2008.
- ^ Cochran, Thomas B. (1997). "Safeguarding nuclear weapons-usable materials in Russia" in International Forum on Illegal Nuclear Traffic., Washington (DC): Natural Resources Defense Council, Inc..
- ^ أ ب Emsley 2001.
- ^ Stockholm International Peace Research Institute 2007, p. 567.
- ^ Poet, S. E.; Martell, EA (October 1972). "Plutonium-239 and americium-241 contamination in the Denver area". Health Physics. 23 (4): 537–48. doi:10.1097/00004032-197210000-00012. PMID 4634934.
- ^ Johnson, C. J. (October 1981). "Cancer Incidence in an area contaminated with radionuclides near a nuclear installation". Ambio. 10 (4): 176–182. JSTOR 4312671. PMID 7348208. Reprinted in Johnson, C. J (Oct 1981). "Cancer Incidence in an area contaminated with radionuclides near a nuclear installation". Colo Med. 78 (10): 385–92. PMID 7348208.
- ^ "Rocky Flats National Wildlife Refuge". U.S. Fish & Wildlife Service. Retrieved 2 July 2013.
- ^ Press Secretary (July 23, 2002). "President Signs Yucca Mountain Bill". Washington (DC): Office of the Press Secretary, White House. Archived from the original on March 6, 2008. Retrieved February 9, 2015.
- ^ Hebert, H. Josef (March 6, 2009). "Nuclear waste won't be going to Nevada's Yucca Mountain, Obama official says". Chicago Tribune. p. 4. Archived from the original on March 24, 2011. Retrieved 2011-03-17.
- ^ "About the Commission". Archived from the original on June 21, 2011.
- ^ أ ب Blue Ribbon Commission on America’s Nuclear Future. "Disposal Subcommittee Report to the Full Commission" (PDF). Archived from the original (PDF) on January 25, 2017. Retrieved February 26, 2017.
- ^ أ ب ت Moss, William; Eckhardt, Roger (1995). "The Human Plutonium Injection Experiments" (PDF). Los Alamos Science. Los Alamos National Laboratory. 23: 188, 205, 208, 214. Retrieved June 6, 2006.
- ^ أ ب Voelz, George L. (2000). "Plutonium and Health: How great is the risk?". Los Alamos Science. Los Alamos (NM): Los Alamos National Laboratory (26): 78–79.
- ^ أ ب Longworth, R. C. (November–December 1999). "Injected! Book review: The Plutonium Files: America's Secret Medical Experiments in the Cold War". The Bulletin of the Atomic Scientists. 55 (6): 58–61. doi:10.2968/055006016.
- ^ Moss, William, and Roger Eckhardt. (1995). "The human plutonium injection experiments." Los Alamos Science. 23: 177–233.
- ^ Openness, DOE. (June 1998). Human Radiation Experiments: ACHRE Report. Chapter 5: The Manhattan district Experiments; the first injection. Washington, DC. Superintendent of Documents US Government Printing Office.
- ^ AEC no. UR-38, 1948 Quarterly Technical Report
- ^ Yesley, Michael S. (1995). "'Ethical Harm' and the Plutonium Injection Experiments" (PDF). Los Alamos Science. 23: 280–283. Retrieved February 15, 2009.
- ^ Much of the information about the plutonium in the Fat Man bomb comes from reports of the criticality accidents of Harry K. Daghlian, Jr. and Louis Slotin, both of whom died after conducting experiments with plutonium bomb cores. See http://members.tripod.com/~Arnold_Dion/Daghlian/accident.html.
- ^ D.C . Hoffman, F. O. Lawrence, J. L. Mewheter, F. M. Rourke: Detection of Plutonium-244 in Nature. In: Nature, Nr. 34, 1971, pp. 132–134
- ^ "ANL human health fact sheet--plutonium" (PDF). Argonne National Laboratory. October 2001. Retrieved 2007-06-16.
- ^ "Radiological control technical training DOE-HDBK-1122-99" (PDF). U.S. Department of Energy.
- ^ Welsome, Eileen (2000). The Plutonium Files: America's Secret Medical Experiments in the Cold War. New York: Random House. pp. p. 17. ISBN 0-385-31954-1.
{{cite book}}:|pages=has extra text (help); Cite has empty unknown parameter:|coauthors=(help) - ^ National Academy of Sciences, Committee on International Security and Arms Control (1994). "Management and Disposition of Excess Weapons Plutonium".
- ^ David Albright and Kevin O'Neill (1999). The Lessons of Nuclear Secrecy at Rocky Flats. ISIS Issue Brief.
- ^ Primer on Spontaneous Heating and Pyrophoricity - Pyrophoric Metals - Plutonium, Department of Energy Handbook DOE-HDBK-1081-94, December 1994. U.S. Department of Energy, Washington, D.C.
وصلات خارجية
- "A Perspective on the Dangers of Plutonium" by Lawrence Livermore National Laboratory
- Collection of articles on plutonium at the Canadian Coalition for Nuclear Responsibility
- The Myth of Plutonium Toxicity
- Criticality Accidents Report Issued
- Nuclear Weapons: Disposal Options for Surplus Weapons-Usable Plutonium
- Unraveling the Phase Diagram of Plutonium **Dead Link**
- Physical, Nuclear, and Chemical, Properties of Plutonium from IEER
- Los Alamos National Laboratory — Plutonium
- It's Elemental — Plutonium
- DOE Plutonium fact sheet (PDF)
- End of the Plutonium Age, D. Samuels, Discover Magazine, vol. 26, no. 11 (November, 2005).
- WebElements.com — Plutonium
- EnvironmentalChemistry.com — Plutonium (JavaScript required)
- Federation of American Scientists — Plutonium production
- nuclearweaponarchive.org — Plutonium Manufacture and Fabrication
- Ambient pressure phase diagram of plutonium — A unified theory for α-Pu and δ-Pu, P. Söderlind, Europhys. Lett., 55 (4), p. 525 (2001).
- Nuclear Files.org Information regarding world plutonium inventories
- "Challenges in Plutonium Science" — Special issue of Los Alamos Science from 2000 dedicated to scientific work on plutonium.
- NLM Hazardous Substances Databank - Plutonium, Radioactive
- Plutonium: A History of the World's Most Dangerous Element
- Annotated bibliography on plutonium from the Alsos Digital Library.
| الجدول الدوري | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||
خطأ استشهاد: وسوم <ref> موجودة لمجموعة اسمها "note"، ولكن لم يتم العثور على وسم <references group="note"/>
- CS1 errors: extra text: pages
- Short description is different from Wikidata
- Pages using infobox element with unknown parameters
- Articles with hatnote templates targeting a nonexistent page
- مقالات ذات عبارات بحاجة لمصادر
- أكتينيدات
- مواد نووية
- پلوتونيوم
- عوامل مسرطنة
- علم سميات العناصر
- عناصر اصطناعية
- عناصر كيميائية
- الصفحات التي تستخدم تنسيقا مهملا للعلامات الكيميائية

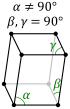

![{\displaystyle {\ce {{^{238}_{92}U}+{^{1}_{0}n}->{^{239}_{92}U}->[\beta ^{-}][23.5\ {\ce {min}}]{^{239}_{93}Np}->[\beta ^{-}][2.3565\ {\ce {d}}]{^{239}_{94}Pu}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f9ba9e4744226a97ce8a41fd5b5e50b18cc259a9)
![{\displaystyle {\begin{aligned}{\ce {{^{238}_{92}U}+{^{2}_{1}D}->}}&{\ce {{^{238}_{93}Np}+2_{0}^{1}n}}\\&{\ce {^{238}_{93}Np->[\beta ^{-}][2.117\ {\ce {d}}]{^{238}_{94}Pu}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1b584c932e52212178b2befe7a512a7b28f87d35)