تترودوتوكسين
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
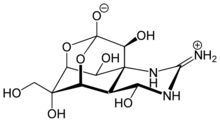
(4R,4aR,5R,6S,7S,8S,8aR,10S,12S)-2-azaniumylidene-4,6,8,12-tetrahydroxy-6-(hydroximethyl)-2,3,4,4a,5,6,7,8-octahydro-1H-8a,10-methano-5,7-(epoxymethanooxy)quinazolin-10-olate
| |
| أسماء أخرى
anhydrotetrodotoxin, 4-epitetrodotoxin, tetrodonic acid, TTX
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.236 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | C11H17N3O8 |
| كتلة مولية | 319.22 g mol-1 |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
تترودوتوكسين، اختصاره TTX، هو سم عصبي ليس له ترياق معروف. نجحت بعض تجارب الترياق على الفئران، لكن لا يوجد تجارب مؤكدة للترياق على البشر.[1] Fampridine has been shown to reverse tetrodotoxin toxicity in animal experiments.[2] Tetrodotoxin blocks action potentials in nerves by binding to the voltage-gated, fast sodium channels in nerve cell membranes, essentially preventing any affected nerve cells from firing by blocking the channels used in the process.[3] The binding site of this toxin is located at the pore opening of the voltage-gated Na+ channel. Its name derives from Tetraodontiformes, the name of the order that includes the pufferfish, porcupinefish, ocean sunfish or mola, and triggerfish, several species of which carry the toxin. Although tetrodotoxin was discovered in these fish and found in several other animals (e.g., blue-ringed octopus, rough-skinned newt,[4] and Naticidae[5]) it is actually produced by certain symbiotic bacteria, such as Pseudoalteromonas tetraodonis, certain species of Pseudomonas and Vibrio, as well as some others that reside within these animals.
Its mechanism of action, selective blocking of the sodium channel, was shown definitively in 1964 by Toshio Narahashi and John W. Moore at Duke University, using Moore's sucrose gap voltage clamp technique.[6]
مصادره في الطبيعة
تم عزل سم التترودوتوكسين من أنواع حيوانية مختلفة، منها جنس تاريشا، وهو أحد أجناس سمندل الماء الغربي (والذي كان يطلق عليه سابقاً اسم "تاريشاتوكسين")، النفيخ، علاجيممن جنس أتلوپس، أنواع مختلفة من الأخطبوط الأزرق الدائري جنس هاپالوشلينا (والتي كانت تسمى "ماكولوتوكسين")، أنواع مختلفة من نجم البحر، وخاصة [[ several sea stars, certain السمكة الملائكية، الديدان المسطحة، أنواع مختلفة من هلبيات الفك (الديدان السهمية)، بعض أنواع خرطوميات الجوف (الديدان الشريطية) وأنواع مختلفة من xanthid crabs.[citation needed] ويستخدم السم على أنه دفاع حيوية لحماية الحيوان من الافتراس، أو للحماية وكسم لتخدير الضحية قبل افتراسها _الأخطبوط، هلبيات الفك والديدان الشريطية). Tarichatoxin and maculotoxin were shown to be identical to tetrodotoxin in 1964 and 1978, respectively.[citation needed] The toxin is produced by bacteria within blue-ringed octopuses.[7] The most common bacteria associated with TTX production are Vibrio bacteria, with Vibrio alginolyticus being the most common species. Pufferfish,[8] chaetognaths,[9] and nemerteans[10] have been shown to contain Vibrio alginolyticus and TTX. The link between these facts and production of TTX in animals has not been firmly established, and there remains much debate in the literature as to whether the bacteria are truly the source of TTX in animals.[11]
الكيمياء الحيوية
Tetrodotoxin binds to what is known as site 1 of the fast voltage-gated sodium channel. Site 1 is located at the extracellular pore opening of the ion channel. The binding of any molecules to this site will temporarily disable the function of the ion channel. Saxitoxin and several of the conotoxins also bind the same site.
The use of this toxin as a biochemical probe has elucidated two distinct types of voltage-gated sodium channels present in humans: the tetrodotoxin-sensitive voltage-gated sodium channel (TTX-s Na+ channel) and the tetrodotoxin-resistant voltage-gated sodium channel (TTX-r Na+ channel). Tetrodotoxin binds to TTX-s Na+ channels with a binding affinity of 5-15 nanomolar, while the TTX-r Na+ channels bind TTX with low micromolar affinity. Nerve cells containing TTX-r Na+ channels are located primarily in cardiac tissue, while nerve cells containing TTX-s Na+ channels dominate the rest of the body. The prevalence of TTX-s Na+ channels in the central nervous system makes tetrodotoxin a valuable agent for the silencing of neural activity within a cell culture.
The toxin blocks the fast Na+ current in human myocytes (the contractile cells of the muscles), thereby inhibiting their contraction. By contrast, the sodium channels in pacemaker cells of the heart are of the slow variety, so action potentials in the cardiac nodes are not inhibited by the compound. The myocytes in the atrium, which surround the main cardiac pacemaker, do express this fast Na+ current and therefore the electrical activity is blocked and the heart fails to beat.
Blocking of fast Na+ channels has potential medical use in treating some cardiac arrhythmias. Tetrodotoxin has proved useful in the treatment of pain (originally used in Japan in the 1930s) from such diverse problems as terminal cancer,[12] migraines, and heroin withdrawal.[13]
التركيب
Yoshito Kishi et al. Nagoya University, Nagoya, Japan, (now at Harvard University) reported the first total synthesis of D,L-tetrodotoxin in 1972.[14][15] M. Isobe et al. at Nagoya University, Japan[16][17][18] and J. Du Bois et al. at Stanford University, U.S., reported the asymmetric total synthesis of tetrodotoxin in 2003.[19] The two 2003 syntheses used very different strategies, with Isobe's route based on a Diels-Alder approach and Du Bois's work using C-H bond activation.
التسمم
Tetrodotoxin is roughly 10 times more poisonous than potassium cyanide.[20] Fish poisoning by consumption of members of the order Tetraodontiformes is extremely serious. The organs (e.g. liver) of the pufferfish can contain levels of tetrodotoxin sufficient to produce paralysis of the diaphragm and death due to respiratory failure.[21] Toxicity varies between species and at different seasons and geographic localities, and the flesh of many pufferfish may not be dangerously toxic. It is not always fatal; but at near-lethal doses, it can leave a person in a state of near-death for several days, while the person remains conscious. For this reason, tetrodotoxin has been alleged an ingredient in Haitian Vodou and the closest approximation of zombieism, an idea popularized by Harvard-trained ethnobotanist Wade Davis in a 1983 paper, and in his 1985 book, The Serpent and the Rainbow. This idea was dismissed by the scientific community in the 1980s, as the descriptions of voodoo zombies do not match the symptoms displayed by victims of tetrodotoxin poisoning, and the alleged incidents of zombies created in this manner could not be substantiated.[22]
السمية
The Material Safety Data Sheet for tetrodotoxin lists the oral median lethal dose (LD50) for mice as 334 μg per kg.[23] Assuming the lethal dose for humans is similar, 25 milligrams (0.000881 oz) of tetrodotoxin would be expected to kill a 75 kg (165 lb) person. The amount needed to reach a lethal dose by injection is much smaller, 8 μg per kg,[24] or a little over one-half milligram (0.00002 oz) to kill a 75 kg (165 lb) person.
التاريخ
كان الكابتن جيمس كوك من أولى الحالات المسجلة للتسمم بواسطة التترودوتوكسين في 7 سبتمبر 1774،[21] حيث سجل كوك عن تسمم بعض أفراد طاقمه بعد تناولهم أسماك استوائية محلية تسمى (النفيخ)، ثم تم تغذية بقايا الطعام للخنازير على ظهر المركب. وشعر الطاقم بالخدر وصعوبة في التنفس، بينما عثر على جميع الخنازير ميتة صباح اليوم التالي. فيما بعد، اتضح أن الطاقم قد تناول كمية معتدلة من التترودوتوكسين، فيما أكل الخنازير الأجزاء المتبقية من الأسماك والتي تحتوي على معظم كميات السموم، مما أدى لتأثرهم بصورة أكبر.
تم فصل السم وتسميته لأول مرة عام 1909 بواسطة العالم الياباني د. يوشيزومي تاهارا.[21]
الأعراض والعلاج
The diagnosis of pufferfish poisoning is based on the observed symptomology and recent dietary history.
Symptoms typically develop within 30 minutes of ingestion, but may be delayed by up to four hours; however, death once occurred within 17 minutes of ingestion.[21] Paresthesia of the lips and tongue is followed by sialorrhea, sweating, headache, weakness, lethargy, incoordination, tremor, paralysis, cyanosis, aphonia, dysphagia, seizures, dyspnea, bronchorrhea, bronchospasm, respiratory failure, coma, and hypotension. Gastroenteric symptoms are often severe and include nausea, vomiting, diarrhea, and abdominal pain. Cardiac arrhythmias may precede complete respiratory failure and cardiovascular collapse.
The first symptom of intoxication is a slight numbness of the lips and tongue, appearing between 20 minutes to three hours after eating poisonous pufferfish.[21] The next symptom is increasing paresthesia in the face and extremities, which may be followed by sensations of lightness or floating. Headache, epigastric pain, nausea, diarrhea, and/or vomiting may occur. Occasionally, some reeling or difficulty in walking may occur. The second stage of the intoxication is increasing paralysis. Many victims are unable to move; even sitting may be difficult. There is increasing respiratory distress. Speech is affected, and the victim usually exhibits dyspnea, cyanosis, and hypotension. Paralysis increases and convulsions, mental impairment, and cardiac arrhythmia may occur. The victim, although completely paralyzed, may be conscious and in some cases completely lucid until shortly before death. Death usually occurs within 4 to 6 hours, with a known range of about 20 minutes to 8 hours.
If the patient survives 24 hours, recovery without any residual effects will usually occur over several days.[25]
Therapy is supportive and based on symptoms, with aggressive early airway management.[21] If ingested, treatment can consist of emptying the stomach, feeding the victim activated charcoal to bind the toxin, and taking standard life-support measures to keep the victim alive until the effect of the poison has worn off.[21] Alpha adrenergic agonists are recommended in addition to intravenous fluids to combat hypotension. Anticholinesterase agents have been used with mixed success. No antidote has been developed and approved for human use; but a monoclonal antibody specific to tetrodotoxin has been developed by USAMRIID and was shown to be effective for reducing lethality in murine tests.[26]
التكرار الجغرافي للسمية
Poisonings from tetrodotoxin have been almost exclusively associated with the consumption of pufferfish from waters of the Indo-Pacific ocean regions. Several reported cases of poisonings, including fatalities, involved pufferfish from the Atlantic Ocean, Gulf of Mexico, and Gulf of California. There have been no confirmed cases of tetrodotoxicity from the Atlantic pufferfish, Sphoeroides maculatus; but in three studies, extracts from fish of this species were highly toxic in mice. Several recent intoxications from these fishes in Florida were due to saxitoxin, which causes paralytic shellfish poisoning with very similar symptoms and signs. The trumpet shell Charonia sauliae has been implicated in food poisonings, and evidence suggests it contains a tetrodotoxin derivative. There have been several reported poisonings from mislabelled pufferfish, and at least one report of a fatal episode in Oregon when an individual swallowed a rough-skinned newt Taricha granulosa.[27]
In 2009, a major scare in the Auckland Region of New Zealand was sparked after several dogs died eating Pleurobranchaea maculata (grey side-gilled seaslug) on beaches.[28] Children and pet owners were asked to avoid beaches, and recreational fishing was also interrupted for a time. After exhaustive analysis, it was found that the sea slugs must have ingested tetrodotoxin.[29]
؛حقائق احصائية
From 1974 through 1983, there were 646 reported cases of pufferfish poisoning in Japan, with 179 fatalities.[citation needed] Statistics from the Tokyo Bureau of Social Welfare and Public Health indicate 20–44 incidents of fugu poisoning per year between 1996 and 2006 in the entire country, leading to 34–64 hospitalizations and 0–6 deaths per year, for an average fatality rate of 6.8%.[30] Of the 23 incidents recorded within Tokyo between 1993 and 2006, only one took place in a restaurant, while the others all involved fishermen eating their catch.[30]
Only a few cases have been reported in the United States, and outbreaks in countries outside the Indo-Pacific area are rare, except in Haiti, where tetrodotoxin is thought by some believers in voodoo mythology[22] to assist the creation of so-called zombie poisons.[31]
Genetic background is not a factor in susceptibility to tetrodotoxin poisoning. This toxicosis may be avoided by not consuming animal species known to contain tetrodotoxin, principally pufferfish; other tetrodotoxic species are not usually consumed by humans. Poisoning from tetrodotoxin is of particular public health concern in Japan, where pufferfish "fugu" is a traditional delicacy. It is prepared and sold in special restaurants where trained and licensed chefs carefully remove the viscera to reduce the danger of poisoning. There is potential for misidentification and mislabelling, particularly of prepared, frozen fish products.
تحليل الطعام
The mouse bioassay developed for paralytic shellfish poisoning (PSP) can be used to monitor tetrodotoxin in pufferfish and is the current method of choice. An HPLC method with post-column reaction with alkali and fluorescence has been developed to determine tetrodotoxin and its associated toxins. The alkali degradation products can be confirmed as their trimethylsilyl derivatives by gas chromatography/mass spectrometry.
اختبار سوائل الجسم
Tetrodotoxin may be quantified in serum, whole blood or urine to confirm a diagnosis of poisoning in hospitalized patients or to assist in the forensic investigation of a case of fatal overdosage. Most analytical techniques involve mass spectrometric detection following gas or liquid chromatographic separation.[32]
التنظيم
في الولايات المتحدة، ظهر التترودوتوكسين في قائمة العناصر المختارة التابعة لوزارة الصحة والخدمات الإنسانية الأمريكية،[33] ويجب على العلماء الذين يقومون بأبحاث باستخدام السم التسجيل في الوزارة. However, investigators possessing less than 100 mg are exempt from regulation.[34]
انظر أيضاً
- Conotoxin
- سم عصبي
- Saxitoxin
- تكتين
- Clairvius Narcisse, a Haitian alleged to have been buried alive under the effect of the drug
- The Serpent and the Rainbow (film)
المصادر
- ^ Rivera, V. R.; Poli, M. A.; Bignami, G. S. (1995). "Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice". Toxicon. 33 (9): 1231–1237. doi:10.1016/0041-0101(95)00060-Y. PMID 8585093.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Octopus Envenomations at eMedicine.com
- ^ Hwang DF, Noguchi T (2007). "Tetrodotoxin poisoning". Adv. Food Nutr. Res. Advances in Food and Nutrition Research. 52: 141–236. doi:10.1016/S1043-4526(06)52004-2. ISBN 978-0-12-373711-3. PMID 17425946.
- ^ Hogan CM (2008-12-02). "Rough-Skinned Newt Taricha granulosa". GlobalTwitcher.com. Retrieved 2009-04-06.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Hwang DF, Tai KP, Chueh CH, Lin LC, Jeng SS (1991). "Tetrodotoxin and derivatives in several species of the gastropod Naticidae". Toxicon. 29 (8): 1019–24. doi:10.1016/0041-0101(91)90084-5. PMID 1949060.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Voltage clamp at Scholarpedia
- ^ Hwang DF, Arakawa O, Saito T, Noguchi T, Simidu U, Tsukamoto K, Shida Y, Hashimoto K (1988). "Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus". Marine Biology. 100 (3): 327–332. doi:10.1007/BF00391147.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Noguchi, T.; Hwang, D.F.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. (1987). "Vibrio alginolyticus, a tetrodotoxin-producing bacterium, in the intestines of the fish Fugu vermicularis vermicularis" (PDF). Marine Biology. 94 (4): 625–630. doi:10.1007/BF00431409. Retrieved 2009-04-06.
- ^ Thuesen EV Kogure K (1989). "Bacterial production of tetrodotoxin in four species of Chaetognatha". Biological Bulletin. 176 (2): 191–194. doi:10.2307/1541587. JSTOR 1541587.
- ^ Carroll, S.; McEvoy, E.G.; Gibson, R. (2003). "The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria". Journal of experimental marine biology and ecology. 288 (1): 51–63. doi:10.1016/S0022-0981(02)00595-6.
- ^ Chau R, Kalaitzis JA, Neilan BA (2011). "On the origins and biosynthesis of tetrodotoxin". Aquatic Toxicology. 104 (1–2): 61–72. doi:10.1016/j.aquatox.2011.04.001. PMID 21543051. Retrieved 2011-07-27.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hagen NA, du Souich P, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Love R, Ngoc AH; on behalf of the Canadian Tetrodotoxin Study Group (2008). "Tetrodotoxin for Moderate to Severe Cancer Pain: A Randomized, Double Blind, Parallel Design Multicenter Study". J Pain Symptom Manage. 35 (4): 420–9. doi:10.1016/j.jpainsymman.2007.05.011. PMID 18243639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stimmel, Barry (2002). "12: Heroin Addiction". Alcoholism, drug addiction, and the road to recovery: life on the edge. New York: Haworth Medical Press. ISBN 0-7890-0553-0.
Tetrodotoxin blocks the sodium currents and is believed to have potential as a potent analgesic and as an effective agent in detoxoification from heroin addiction without withdrawal symptoms and without producing physical dependence
- ^ Kishi Y, Aratani M, Fukuyama T, Nakatsubo F, Goto T, Inoue S, Tanino H, Sugiura S, Kakoi H (1972). "Synthetic studies on tetrodotoxin and related compounds. 3. A stereospecific synthesis of an equivalent of acetylated tetrodamine". J. Am. Chem. Soc. 94 (26): 9217–9. doi:10.1021/ja00781a038. PMID 4642370.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kishi Y, Fukuyama T, Aratani M, Nakatsubo F, Goto T, Inoue S, Tanino H, Sugiura S, Kakoi H (1972). "Synthetic studies on tetrodotoxin and related compounds. IV. Stereospecific total syntheses of DL-tetrodotoxin". J. Am. Chem. Soc. 94 (26): 9219–9221. doi:10.1021/ja00781a039. PMID 4642371.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ohyabu N, Nishikawa T, Isobe M (2003). "First Asymmetric Total Synthesis of Tetrodotoxin". J. Am. Chem. Soc. 125 (29): 8798–8805. doi:10.1021/ja0342998. PMID 12862474.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nishikawa T, Urabe D, Isobe M (2004). "An Efficient Total Synthesis of Optically Active Tetrodotoxin". Angewandte Chemie International Edition. 43 (36): 4782–4785. doi:10.1002/anie.200460293. PMID 15366086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Douglass Taber (2005-05-02). "Synthesis of (-)-Tetrodotoxin". Organic Chemistry Portal. organic-chemistry.org. Retrieved 2008-05-29.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Hinman A, Du Bois J (2003). "A Stereoselective Synthesis of (-)-Tetrodotoxin". J. Am. Chem. Soc. 125 (38): 11510–11511. doi:10.1021/ja0368305. PMID 13129349.
- ^ Reference to 100 times more poisonous.
- ^ أ ب ت ث ج ح خ Clark RF, Williams SR, Nordt SP, Manoguerra AS (1999). "A review of selected seafood poisonings". Undersea Hyperb Med. 26 (3): 175–84. PMID 10485519. Retrieved 2008-08-12.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ أ ب Hines, Terence; "Zombies and Tetrodotoxin"; Skeptical Inquirer; May/June 2008; Volume 32, Issue 3; pp. 60–62.
- ^ Material Safety Data Sheet Tetrodotoxin ACC# 01139 https://fscimage.fishersci.com/msds/01139.htm
- ^ Material Safety Data Sheet Tetrodotoxin. Sigma-Aldrich Version 1.6 updated 10 March 2007.
- ^ Toxicity, Tetrodotoxin -Theodore I Benzer, MD, PhD
- ^ Victor R. Rivera, Mark A. Poli, Gary S. Bignami, Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice, Toxicon, Volume 33, Issue 9, September 1995, Pages 1231-1237, ISSN 0041-0101, 10.1016/0041-0101(95)00060-Y. (http://www.sciencedirect.com/science/article/pii/004101019500060Y)
- ^ Bradley, S. G., and L. J. Klika. 1981. A fatal poisoning from the Oregon rough-skinned newt (Taricha granulosa). JAMA: The Journal of the American Medical Association 246:247.
- ^ McNabb, P.; Mackenzie, L.; Selwood, A.; Rhodes, L.; Taylor, D.; Cornelison, C. (2009). Review of tetrodotoxins in the sea slug Pleurobranchaea maculata and coincidence of dog deaths along Auckland beaches. Prepared by Cawthron Institute for the Auckland Regional Council. Auckland Regional Council Technical Report 2009/ 108.
- ^ Gibson, Eloise (15 August 2009). "Puffer fish toxin blamed for deaths of two dogs". The New Zealand Herald. Retrieved 19 November 2011.
- ^ أ ب 危険がいっぱい ふぐの素人料理 東京都福祉保健局
- ^ Anderson WH (1988). "Tetrodotoxin and the zombie phenomenon". Journal of Ethnopharmacology. 23 (1): 121–6. doi:10.1016/0378-8741(88)90122-5. PMID 3419200.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1521–1522.
- ^ HHS and USDA Select Agents and Toxins 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73. http://www.cdc.gov/od/sap/docs/salist.pdf
- ^ National Select Agent Registry http://www.selectagents.gov/Permissible%20Toxin%20Amounts.html
وصلات خارجية
- MeSH Tetrodotoxin
- Tetrodotoxin: essential data (1999)
- Tetrodotoxin from the Bad Bug Book at the U.S. Food and Drug Administration website
- New York Times, "Whatever Doesn't Kill Some Animals Can Make Them Deadly"
- U.S. National Library of Medicine: Hazardous Substances Databank – Tetrodotoxin
- CS1 errors: unsupported parameter
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed KEGG identifier
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- Articles with unsourced statements from November 2008
- Articles with unsourced statements from April 2008
- سموم عصبية
- سموم القناة الشاردية
- Guanidines
- أسلحة بيولوجية سامة
- كحول
- سموم الفقاريات