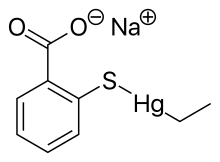
ثيومرسال
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Ethyl(2-mercaptobenzoato-(2-)-O,S) mercurate(1-) sodium
| |
| أسماء أخرى
Mercury((o-carboxyphenyl)thio)ethyl sodium salt, sodium ethylmercurithiosalicylate
| |
| المُعرِّفات | |
| رقم CAS | |
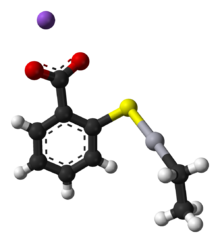
3D model (JSmol)
|
|
| مرجع بايلستاين | 8169555 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.192 |
| رقم EC |
|
| مرجع Gmelin | 1677155 |
| KEGG | |
PubChem CID
|
|
| رقم RTECS |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | C9H9HgNaO2S |
| كتلة مولية | 404.81 g/mol |
| المظهر | White or slightly yellow powder |
| الكثافة | 2.508 g/cm3[1] |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | 1000 g/L (20 °C) |
| علم الأدوية | |
| D08AK06 (WHO) | |
| المخاطر | |
| صفحة بيانات السلامة | External MSDS |
| ن.م.ع. مخطط تصويري |   
|
| ن.م.ع. كلمة الاشارة | Danger |
| H300, H310, H330, H373, H410 | |
| P260, P273, P280, P301, P302, P304, P310, P330, P340, P352[2] | |
| NFPA 704 (معيـَّن النار) | |
| نقطة الوميض | 250 °C (482 °F; 523 K) |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
75 mg/kg (oral, rat)[3] |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Thiomersal (INN), or thimerosal (USAN, JAN), is an organomercury compound. It is a well-established antiseptic and antifungal agent.[4]
The pharmaceutical corporation Eli Lilly and Company gave thiomersal the trade name Merthiolate. It has been used as a preservative in vaccines, immunoglobulin preparations, skin test antigens, antivenins, ophthalmic and nasal products, and tattoo inks.[5] In spite of the scientific consensus that fears about its safety are unsubstantiated,[6][7][8][9] its use as a vaccine preservative has been called into question by anti-vaccination groups. Due to this public pressure the substance was phased out of routine childhood vaccines in the United States, the European Union, and a few other countries in response to popular fears.[10] It remains in use as a preservative for annual flu vaccines.[11]
التاريخ
Morris Kharasch, a chemist then at the University of Maryland filed a patent application for thiomersal in 1927;[12] Eli Lilly later marketed the compound under the trade name Merthiolate.[13] In vitro tests conducted by Lilly investigators H. M. Powell and W. A. Jamieson found that it was forty to fifty times as effective as phenol against Staphylococcus aureus.[13] It was used to kill bacteria and prevent contamination in antiseptic ointments, creams, jellies, and sprays used by consumers and in hospitals, including nasal sprays, eye drops, contact lens solutions, immunoglobulins, and vaccines. Thiomersal was used as a preservative (bactericide) so that multidose vials of vaccines could be used instead of single-dose vials, which are more expensive. By 1938, Lilly's assistant director of research listed thiomersal as one of the five most important drugs ever developed by the company.[13]
Structure
Thiomersal features mercury(II) with a coordination number 2, i.e. two ligands are attached to Hg, the thiolate and the ethyl group. The carboxylate group confers solubility in water. Like other two-coordinate Hg(II) compounds, the coordination geometry of Hg is linear, with a 180° S-Hg-C angle. Typically, organomercury thiolate compounds are prepared from organomercury chlorides.[1]
Uses
Thiomersal's main use is as an antiseptic and antifungal agent, due to the oligodynamic effect. In multidose injectable drug delivery systems, it prevents serious adverse effects such as the Staphylococcus infection that, in one 1928 incident, killed 12 of 21 children vaccinated with a diphtheria vaccine that lacked a preservative.[14] Unlike existing preservatives at the time, such as phenol and cresol, thiomersal does not reduce the potency of the vaccines that it protects.[13] Bacteriostatics such as thiomersal are not needed in single-dose injectables.[15]
In the United States, countries in the European Union, and a few other affluent countries, thiomersal is no longer used as a preservative in routine childhood vaccination schedules.[10] In the U.S., all vaccines routinely recommended for children 6 years of age and younger are available in formulations that do not contain thimerosal. There are only two vaccines (a TD and the single-dose version of the trivalent influenza vaccine Fluvirin) which may contain a trace of thiomersal from steps in manufacture, i.e. less than 1 micrograms of mercury per dose.[14] The multi-dose versions of some trivalent and quadrivalent influenza vaccines can contain up to 25 micrograms of mercury per dose from thiomersal. Also, four rarely used treatments for pit viper, coral snake, and black widow venom still contain thiomersal.[16]
Outside North America and Europe, many vaccines contain thiomersal; the World Health Organization has concluded that there is no evidence of toxicity from thiomersal in vaccines and no reason on safety grounds to change to more expensive single-dose administration.[17] The United Nations Environment Program backed away from an earlier proposal of putting thiomersal, when used as a component in vaccines, on the list of banned compounds, in its campaign to reduce exposure to mercury worldwide.[18] Citing that eliminating the preservative in multi-dose vaccines, primarily used in developing countries, would lead to high cost and a requirement for refrigeration which the developing countries can ill afford, the UN's final decision at the Minamata Convention on Mercury in 2013 was to exclude thiomersal from the treaty.[19]
Toxicology
General toxicity
Thiomersal is very toxic by inhalation, ingestion, and in contact with skin (EC hazard symbol T+), with a danger of cumulative effects. It is also very toxic to aquatic organisms and may cause long-term adverse effects in aquatic environments (EC hazard symbol N).[20] In the body, it is metabolized or degraded to ethylmercury (C2H5Hg+) and thiosalicylate.[14]
Cases have been reported of severe mercury poisoning by accidental exposure or attempted suicide, with some fatalities.[21] Animal experiments suggest that thiomersal rapidly dissociates to release ethylmercury after injection; that the disposition patterns of mercury are similar to those after exposure to equivalent doses of ethylmercury chloride; and that the central nervous system and the kidneys are targets, with lack of motor coordination being a common sign. Similar signs and symptoms have been observed in accidental human poisonings. The mechanisms of toxic action are unknown.[21]
Fecal excretion accounts for most of the elimination from the body. Ethylmercury clears from blood with a half-life of about 18 days in adults by breakdown into other chemicals, including inorganic mercury.[22] Ethylmercury is eliminated from the brain in about 14 days in infant monkeys. Risk assessment for effects on the nervous system have been made by extrapolating from dose-response relationships for methylmercury.[23] Methylmercury and ethylmercury distribute to all body tissues, crossing the blood–brain barrier and the placental barrier, and ethylmercury also moves freely throughout the body.[24]
Concerns based on extrapolations from methylmercury caused thiomersal to be removed from U.S. childhood vaccines, starting in 1999. Since then, it has been found that ethylmercury is eliminated from the body and the brain significantly faster than methylmercury, so the late-1990s risk assessments turned out to be overly conservative.[23] Though inorganic mercury metabolized from ethylmercury has a much longer half-life in the brain, at least 120 days, it appears to be much less toxic than the inorganic mercury produced from mercury vapor, for reasons not yet understood.[23]
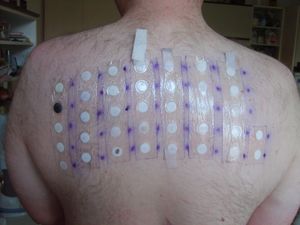
As an allergen
Thiomersal is used in patch testing for people who have dermatitis, conjunctivitis, and other potentially allergic reactions. A 2007 study in Norway found that 1.9% of adults had a positive patch test reaction to thiomersal;[25] a higher prevalence of contact allergy (up to 6.6%) was observed in German populations.[26] Thiomersal-sensitive individuals can receive intramuscular rather than subcutaneous immunization,[27] though there have been no large sample sized studies regarding this matter to date. In real-world practice on vaccination of adult populations, contact allergy does not seem to elicit clinical reaction.[26]
Thiomersal allergy has decreased in Denmark, probably because of its exclusion from vaccines there.[28] In a recent study of Polish children and adolescents with chronic/recurrent eczema, positive reactions to thiomersal were found in 11.7% of children (7–8 y.o.) and 37.6% of adolescents (16–17 y.o.). This difference in the sensitization rates can be explained by changing exposure patterns: The adolescents have received six thiomersal-preserved vaccines during their life course, with the last immunization taking place 2–3 years before the mentioned study, younger children received only four thiomersal-preserved vaccines, with the last one applied five years before the study, while further immunizations were performed with new thiomersal-free vaccines.[29]
Disproven autism hypothesis
Following a review of mercury-containing food and drugs mandated in 1999, the Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics asked vaccine manufacturers to remove thiomersal from vaccines as a purely precautionary measure, and it was rapidly phased out of most U.S. and European vaccines.[13][30] Many parents saw the action to remove thiomersal—in the setting of a perceived increasing rate of autism as well as increasing number of vaccines in the childhood vaccination schedule—as indicating that the preservative was the cause of autism.[13] The scientific consensus is that there is no evidence supporting these claims, and the rate of autism continues to climb despite elimination of thiomersal from routine childhood vaccines.[8][31][32][33] Major scientific and medical bodies such as the Institute of Medicine[33] and World Health Organization,[34][35] as well as governmental agencies such as the Food and Drug Administration[14] and the CDC[36] reject any role for thiomersal in autism or other neurodevelopmental disorders.[37] This controversy has caused harm due to parents attempting to treat their autistic children with unproven and possibly dangerous treatments, discouraging parents from vaccinating their children due to fears about thiomersal toxicity,[38] and diverting resources away from research into more promising areas for the cause of autism.[39] Thousands of lawsuits have been filed in a U.S. federal court to seek damages from alleged toxicity from vaccines, including those purportedly caused by thiomersal.[40]
انظر أيضاً
- Nitromersol, a related antimicrobial
- Phenylmercuric nitrate - an organomercury compound with powerful antiseptic and antifungal effects
المراجع
- ^ أ ب Melnick, J. G.; Yurkerwich, K.; Buccella, D.; Sattler, W.; Parkin, G. (2008). "Molecular Structures of Thimerosal (Merthiolate) and Other Arylthiolate Mercury Alkyl Compounds". Inorg. Chem. 47 (14): 6421–26. doi:10.1021/ic8005426. PMID 18533648.
- ^ "Thimerosal T5125".
- ^ Chambers, Michael. "ChemIDplus – 54-64-8 – RTKIYNMVFMVABJ-UHFFFAOYSA-L – Thimerosal [USP:JAN] – Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. Retrieved 3 أبريل 2018.
- ^ "Thimerosal and Vaccines | Vaccine Safety | CDC". Centers for Disease Control and Prevention (in الإنجليزية الأمريكية). 25 أغسطس 2020. Retrieved 4 مايو 2023.
- ^ Sharpe, M. A.; Livingston, A. D.; Baskin, D. S. (2012). "Thimerosal-Derived Ethylmercury is a Mitochondrial Toxin in Human Astrocytes: Possible Role of Fenton Chemistry in the Oxidation and Breakage of mtDNA". Journal of Toxicology. 2012: 1–12. doi:10.1155/2012/373678. PMC 3395253. PMID 22811707.
...widely used in medical products, including as a preservative in vaccines, immunoglobulin preparations, skin test antigens, antivenins, ophthalmic and nasal products, and tattoo inks...
- ^ Immunization Safety Review Committee, Board on Health Promotion and Disease Prevention, Institute of Medicine (2004). Immunization Safety Review: Vaccines and Autism. Washington, DC: The National Academies Press. ISBN 978-0-309-09237-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Doja, Asif; Roberts, Wendy (نوفمبر 2006). "Immunizations and autism: a review of the literature". Can J Neurol Sci. 33 (4): 341–46. doi:10.1017/s031716710000528x. PMID 17168158.
- ^ أ ب "Vaccines Do Not Cause Autism". cdc.gov. Retrieved 29 نوفمبر 2015.
- ^ Gołoś, A; Lutyńska, A (2015). "Thiomersal-containing vaccines - a review of the current state of knowledge". Przeglad Epidemiologiczny. 69 (1): 59–64, 157–61. PMID 25862449.
- ^ أ ب Bigham, M; Copes, R (2005). "Thiomersal in vaccines: balancing the risk of adverse effects with the risk of vaccine-preventable disease". Drug Saf. 28 (2): 89–101. doi:10.2165/00002018-200528020-00001. PMID 15691220. S2CID 11570020.
- ^ "Not Immune". The New Yorker (in الإنجليزية الأمريكية). 8 فبراير 2015. Retrieved 2 أكتوبر 2022.
- ^ U.S. Patent 1٬672٬615 "Alkyl mercuric sulphur compound and process of producing it".
- ^ أ ب ت ث ج ح Baker, JP (2008). "Mercury, Vaccines, and Autism: One Controversy, Three Histories". Am J Public Health. 98 (2): 244–53. doi:10.2105/AJPH.2007.113159. PMC 2376879. PMID 18172138.
- ^ أ ب ت ث "Thimerosal and vaccines" (in الإنجليزية). Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2 يناير 2018. Retrieved 9 أبريل 2023.
- ^ "Thimerosal in Vaccines: Frequently Asked Questions". Food and Drug Administration. Retrieved 9 مارس 2008.
- ^ "Mercury in plasma-derived products". U.S. Food and Drug Administration. 9 سبتمبر 2004. Archived from the original on 29 سبتمبر 2007. Retrieved 1 أكتوبر 2007.
- ^ Global Advisory Committee on Vaccine Safety (14 يوليو 2006). "Thiomersal and vaccines". World Health Organization. Archived from the original on 20 أغسطس 2003. Retrieved 20 نوفمبر 2007.
- ^ Hamilton, Jon (17 ديسمبر 2012). "Doctors Argue Against Proposed Ban on Vaccine Preservative". NPR. Retrieved 25 فبراير 2013.
- ^ Sulaski Wyckoff, Alyson (22 يناير 2013). "Global ban on mercury grants exception to thimerosal-containing vaccines". AAP News. American Academy of Pediatrics. Retrieved 24 أغسطس 2019.
- ^ "Safety data sheet, Thiomersal Ph Eur, BP, USP" (PDF). Merck. 12 يونيو 2005. Retrieved 1 يناير 2010.
- ^ أ ب Clarkson, TW (2002). "The three modern faces of mercury". Environ Health Perspect. 110 (S1): 11–23. doi:10.1289/ehp.02110s111. PMC 1241144. PMID 11834460. Archived from the original on 6 سبتمبر 2008.
- ^ Magos, L. (2003). "Neurotoxic character of thimerosal and the allometric extrapolation of adult clearance half-time to infants". Journal of Applied Toxicology. 23 (4): 263–269. doi:10.1002/jat.918. PMID 12884410. S2CID 20703489. Retrieved 19 يناير 2021.
- ^ أ ب ت Clarkson, TW; Magos, L (2006). "The toxicology of mercury and its chemical compounds". Crit Rev Toxicol. 36 (8): 609–62. doi:10.1080/10408440600845619. PMID 16973445. S2CID 37652857.
- ^ Clarkson, TW; Vyas, JB; Ballatori, N (2007). "Mechanisms of mercury disposition in the body". Am J Ind Med. 50 (10): 757–64. doi:10.1002/ajim.20476. PMID 17477364.
- ^ Dotterud, LK; Smith-Sivertsen, T (2007). "Allergic contact sensitization in the general adult population: a population-based study from Northern Norway". Contact Dermatitis. 56 (1): 10–15. doi:10.1111/j.1600-0536.2007.00980.x. PMID 17177703. S2CID 25765635.
- ^ أ ب Uter, W; Ludwig, A; Balda, BR (2004). "The prevalence of contact allergy differed between population-based and clinic-based data". J Clin Epidemiol. 57 (6): 627–32. doi:10.1016/j.jclinepi.2003.04.002. PMID 15246132.
- ^ Aberer, W (1991). "Vaccination despite thimerosal sensitivity". Contact Dermatitis. 24 (1): 6–10. doi:10.1111/j.1600-0536.1991.tb01621.x. PMID 2044374. S2CID 43264826.
- ^ Thyssen, JP; Linneberg, A; Menné, T; Johansen, JD (2007). "The epidemiology of contact allergy in the general population – prevalence and main findings". Contact Dermatitis. 57 (5): 287–99. doi:10.1111/j.1600-0536.2007.01220.x. PMID 17937743. S2CID 44890665.
- ^ Czarnobilska, E; Obtulowicz, K; Dyga, W; Spiewak, R (2011). "The most important contact sensitizers in Polish children and adolescents with atopy and chronic recurrent eczema as detected with the extended European Baseline Series". Pediatr Allergy Immunol. 22 (2): 252–56. doi:10.1111/j.1399-3038.2010.01075.x. PMID 20969635. S2CID 22195669.
- ^ "Thimerosal in vaccines: frequently asked questions (FAQs)". Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 7 يونيو 2007. Retrieved 22 يوليو 2008.
- ^ DeStefano, F (2007). "Vaccines and autism: evidence does not support a causal association". Clin Pharmacol Ther. 82 (6): 756–59. doi:10.1038/sj.clpt.6100407. PMID 17928818. S2CID 12872702.
- ^ Doja, A; Roberts, W (2006). "Immunizations and autism: a review of the literature". Can J Neurol Sci. 33 (4): 341–46. doi:10.1017/s031716710000528x. PMID 17168158.
- ^ أ ب Immunization Safety Review Committee, Board on Health Promotion and Disease Prevention, Institute of Medicine (2004). Immunization Safety Review: Vaccines and Autism. Washington, DC: The National Academies Press. ISBN 978-0-309-09237-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ World Health Organization (2006). "Thiomersal and vaccines: questions and answers". Archived from the original on 12 أكتوبر 2003. Retrieved 19 مايو 2009.
- ^ WHO. "Statement on thiomersal". www.who.int. Archived from the original on 29 أكتوبر 2012. Retrieved 3 أبريل 2018.
- ^ Centers for Disease Control (8 فبراير 2008). "Mercury and vaccines (thimerosal)". Archived from the original on 18 أبريل 2018. Retrieved 19 مايو 2009.
- ^ Sugarman, SD (2007). "Cases in vaccine court – legal battles over vaccines and autism". N Engl J Med. 357 (13): 1275–77. doi:10.1056/NEJMp078168. PMID 17898095.
- ^ Harris, G; O'Connor, A (25 يونيو 2005). "On autism's cause, it's parents vs. research". New York Times. Retrieved 11 مارس 2016.
- ^ Offit, PA (2007). "Thimerosal and vaccines – a cautionary tale". N Engl J Med. 357 (13): 1278–79. doi:10.1056/NEJMp078187. PMID 17898096.
- ^ Autism cases in vaccine court:
- Sugarman, SD (2007). "Cases in vaccine court – legal battles over vaccines and autism". N Engl J Med. 357 (13): 1275–77. doi:10.1056/NEJMp078168. PMID 17898095.
- U.S. Court of Federal Claims (28 سبتمبر 2007). "Vaccine Program/Office of Special Masters Omnibus Autism Proceeding". Archived from the original on 23 أكتوبر 2007. Retrieved 24 نوفمبر 2007.
- CS1 الإنجليزية الأمريكية-language sources (en-us)
- Short description is different from Wikidata
- Use dmy dates from August 2021
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Articles with hatnote templates targeting a nonexistent page
- Thiolates
- Eli Lilly and Company brands
- Organomercury compounds
- Excipients
- Organic sodium salts
- Benzoates