كينين
 | |
 | |
| البيانات السريرية | |
|---|---|
| فئة السلامة أثناء الحمل | |
| مسارات الدواء | فموي، في الوريد |
| رمز ATC | |
| بيانات الحركية الدوائية | |
| التوافر الحيوي | 76 to 88% |
| ارتباط الپروتين | ~70% |
| الأيض | Hepatic (mostly CYP3A4 and CYP2C19-mediated) |
| Elimination half-life | ~18 ساعة |
| الإخراج | بولي (20%) |
| المعرفات | |
| |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.550 |
| Chemical and physical data | |
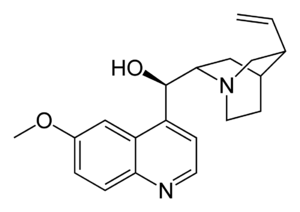
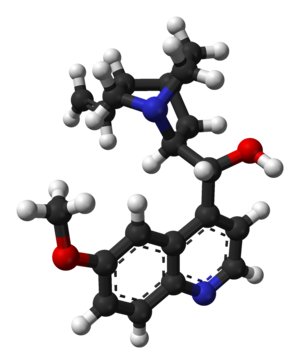
| التركيب | C20H24N2O2 |
| الكتلة المولية | 324.417 g/mol |
| Melting point | 177 °C (351 °F) |
كينين Quinine ({{IPAEng|ˈkwaɪnaɪn (US), kwɪˈniːn, ˈkwɪniːn (GB)({{ هو natural أبيض بللورى قلويد يمتلك مسكن وخافض للحرارة، مضاد الملاريا، مسكن (قاتل للألم)، ومضاد للإلتهاب خواص ذو طعم مر. وهو stereoisomer للكينيدين.
الكينين هو أول دواء فعال أستخدم لعلاج الملاريا malaria التى تسببها بلازموديوم فالسيبارم Plasmodium falciparum, ظهر في قوائم العلاجات في القرن السابع عشر.,وبقى العلاج المفضل لعلاج الملاريا , حتى عام 1940 حيث أستبدل بأدوية أخرى .ومنذ ذلك الحين فإن أدوية عديدة لعلاج الملاريا قد أستحدثت ولكن بقى الكينين مستعملا لعلاج المرض, في بعض الأحيان الحرجة , الكينين يتوافر صرفه بوصفة طبية في الولايات المتحدة الأميريكية.[بحاجة لمصدر] ويستعمل الكينين أيضا لعلاج الليلى تقلص العضلة و إلتهاب المفاصل, و هناك محاولات (بنجاحات بسيطة) لعلاج مرض prion . وقد كان يوما شيئا شائعا غش هيروين وهو الآن ليس شائعا في العالم, بالرغم أن بعض البلدان (مثل سكوتلاندا) مازال لديها هيروين مغشوش بالكينين يباع في الشوارع. [بحاجة لمصدر]
أكتشف أساسا بواسطة هنود كتشوا في بيرو، فإن القلف السنكونا استقدمه الى أوروبا اليسوعيون.
التركيب الكيميائى
يحتوى الكينين على إثنان من الحلقات الكبرى المندمجة: العطرية الكينولين ثنائى الحلقات quinuclidine.
نظرية العمل ضد بلازموديوم فاليسبارم
يعمل الدواء بتثبيط عمل hemozoin biocrystallization, و هكذا يسهل تجميع cytotoxic heme. جزىء الهيم الحر السام يتركز في الطفيل, موديا الى القضاء عليه.
التاريخ
الكينين يعد دواءا مثاليا لإرخاء العضلات, أستعمل فترة طويلة بواسطةQuechua هنود Peru لعلاج الرعشة التى تجتاح الجسم من التعرض للبرد. و صنع من قلف أشجار cinchona, وخلط البيروفيون القلف , مع ماء مسكر للتغلب على مرارة المادة الفعالة, وهكذا صنعوا مشوب مقوى tonic water.
Quinine has been used in un-extracted form by Europeans since at least the early 1600s. Quinine was first used to treat malaria in Rome in 1631. During the 1600s, malaria was endemic to the swamps and marshes surrounding the city of Rome. Over time, malaria was responsible for the death of several popes, many cardinals and countless common citizens of Rome. Most of the priests trained in Rome had seen malaria victims and were familiar with the shivering brought on by the cold phase of the disease. The Jesuit brother Agostino Salumbrino (1561-1642), an apothecary by training who lived in Lima, observed the Quechua using the quinine-containing bark of the cinchona tree for that purpose. While its effect in treating malaria (and hence malaria-induced shivering) was entirely unrelated to its effect in controlling shivering from cold, it was still the correct medicine for malaria. At the first opportunity, he sent a small quantity to Rome to test in treating malaria. In the years that followed, cinchona bark became one of the most valuable commodities shipped from Peru to Europe.
The correct form of quinine best used to treat malaria was found by Charles Marie de La Condamine in 1737. Quinine was isolated and named in 1817 by French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou. The name was derived from the original Quechua (Inca) word for the cinchona tree bark, "quina" or "quina-quina", which roughly means "bark of bark" or "holy bark". Prior to 1820, the bark was first dried, ground to a fine powder and then mixed into a liquid (commonly wine) which was then drunk. Large scale use of quinine as a prophylaxis started around 1850.
Quinine also played a significant role in the colonization of Africa by Europeans. As the harbinger of modern pharmacology, quinine was the prime reason Africa ceased to be known as the white man's grave. A historian has stated that "it was quinine's efficacy that gave colonists fresh opportunities to swarm into the Gold Coast, Nigeria and other parts of west Africa".[1]
To maintain their monopoly on cinchona bark, Peru and surrounding countries began outlawing the exportation of cinchona seeds and saplings beginning in the early 19th century. The Dutch government persisted in their attempts to smuggle the seeds, and by the 1930s Dutch plantations in Java were producing 22 million pounds of cinchona bark, or 97% of the world's quinine production.[1] During الحرب العالمية الثانية, Allied powers were cut off from their supply of quinine when the Germans conquered Holland and the Japanese controlled الفلپين and Indonesia. The الولايات المتحدة, however, had managed to obtain four million cinchona seeds from the Philippines and begin operation of cinchona plantations in Costa Rica. It had come too late, however, and an estimated 60,000 US troops in Africa and the South Pacific died as a result of the lack of quinine.[1]
كينين صناعي
Cinchona trees remain the only practical source of quinine. However, under wartime pressure, research towards its artificial production was undertaken. A formal chemical synthesis was accomplished in 1944 by American chemists R.B. Woodward and W.E. Doering.[2] Since then, several more efficient quinine total syntheses have been achieved,[3] but none of them can compete in economic terms with isolation of the alkaloid from natural sources. The first synthetic organic dye, mauveine, was discovered by William Henry Perkin in 1856 while he was attempting to synthesize quinine.
الجرعة
Quinine is a basic amine and is therefore always presented as a salt. Various preparations that exist include the hydrochloride, dihydrochloride, sulfate, bisulfate and gluconate. This makes quinine dosing very complicated, because each of the salts has a different weight.
The following amounts of each form are equal:
- quinine base 100 mg
- quinine bisulfate 169 mg
- quinine dihydrochloride 122 mg
- quinine hydrochloride 111 mg
- quinine sulfate (actually (quinine)2H2SO4∙2H2O) 121 mg
- quinine gluconate 160 mg.
All quinine salts may be given orally or intravenously (IV); quinine gluconate may also be given intramuscularly (IM) or rectally (PR).[4][5] The main problem with the rectal route is that the dose can be expelled before it is completely absorbed; this can be corrected by giving a half dose again.
The IV dose of quinine is 8 mg/kg of quinine base every eight hours; the IM dose is 12.8 mg/kg of quinine base twice daily; the PR dose is 20 mg/kg of quinine base twice daily. Treatment should be given for seven days.
The preparations available in the UK are quinine sulfate (200 mg or 300 mg tablets) and quinine hydrochloride (300 mg/ml for injection). Quinine is not licensed for IM or PR use in the UK. The adult dose in the UK is 600 mg quinine dihydrochloride IV or 600 mg quinine sulfate orally every eight hours. For nocturnal leg cramps, the dosage is 200-300mg at night.[6]
In the United States, quinine sulfate is available as 324-mg tablets under the brand name Qualaquin; the adult dose is two tablets every eight hours. There is no injectable preparation of quinine licensed in the U.S.: quinidine is used instead.[7][8]
Quinine is not recommended for malaria prevention (prophylaxis) because of its side-effects and poor tolerability, not because it is ineffective. When used for prophylaxis, the dose of quinine sulfate is 300-324mg once daily, starting one week prior to travel and continuing for four weeks after returning.
الأعراض الجانبية
- See: cinchonism
It is usual for quinine in therapeutic doses to cause cinchonism; in rare cases, it may even cause death (usually by pulmonary edema). The development of mild cinchonism is not a reason for stopping or interrupting quinine therapy and the patient should be reassured. Blood glucose levels and electrolyte concentrations must be monitored when quinine is given by injection; the patient should also ideally be in cardiac monitoring when the first quinine injection is given (these precautions are often unavailable in developing countries where malaria is most a problem).
Cinchonism is much less common when quinine is given by mouth, but oral quinine is not well tolerated (quinine is exceedingly bitter and many patients will vomit after ingesting quinine tablets): Other drugs such as Fansidar (sulfadoxine (sulfonamide antibiotic) with pyrimethamine) or Malarone (proguanil with atovaquone) are often used when oral therapy is required. Blood glucose, electrolyte and cardiac monitoring are not necessary when quinine is given by mouth.
Quinine can cause paralysis if accidentally injected into a nerve. It is extremely toxic in overdose, and the advice of a poisons specialist should be sought immediately.
Quinine in some cases can lead to constipation[9], erectile dysfunction, and a loose stool or in rare cases many loose stools.[بحاجة لمصدر]
Abortifacient
Despite popular belief, quinine is an ineffective abortifacient (in the US, quinine is listed as Pregnancy category C [1]). Pregnant women who take toxic doses of quinine will suffer from renal failure before experiencing any kind of quinine-induced abortion.[10]
التفاعل مع الأمراض
Quinine can cause hemolysis in G6PD deficiency, but again this risk is small and the physician should not hesitate to use quinine in patients with G6PD deficiency when there is no alternative. Quinine can also cause drug-induced immune thrombocytopenic purpura (ITP).
Quinine can cause abnormal heart rhythms and should be avoided if possible in patients with atrial fibrillation, conduction defects or heart block.
Quinine can worsen hemoglobinuria, myasthenia gravis and optic neuritis.[بحاجة لمصدر]
الإضرار بالسمع
Some studies have related the use of quinine and hearing impairment, in particular high-frequency loss, but it has not been conclusively established whether such impairment is temporary or permanent.[11]
Regulation by the United States Food and Drug Administration
From 1969 to 1992, the U.S. Food and Drug Administration (FDA) received 157 reports of health problems related to quinine use, including 23 which had resulted in death.[12] In 1994, the FDA banned the use of over-the-counter (OTC) quinine as a treatment for nocturnal leg cramps. Pfizer Pharmaceuticals had been selling the brand name Legatrin for this purpose. Doctors may still prescribe quinine, but the FDA has ordered firms to stop marketing unapproved drug products containing quinine. As of 2008, pharmacists will not sell quinine even if the patient has used a prescription for it in the past.[بحاجة لمصدر] The FDA is also cautioning consumers about off-label use of quinine to treat leg cramps. Quinine is approved for treatment of malaria, but is also commonly prescribed to treat leg cramps and similar conditions. Because malaria is life-threatening, the risks associated with quinine use are considered acceptable when used to treat that condition. However, because of the drug's risks the FDA believes it should not be used to prevent or treat leg cramps.[13]
الاستخدامات غير الطبية للكينين
Quinine is a flavor component of tonic water and bitter lemon. According to tradition, the bitter taste of anti-malarial quinine tonic led British colonials in الهند to mix it with gin, thus creating the gin and tonic cocktail, which is still popular today in many parts of the world, especially the U.K., United States, southern Canada, parts of Australia and even Lhasa, Tibet.
Bark of Remijia contains 0.5 - 2 % of quinine. The bark is cheaper than bark of Cinchona and as it has an intense taste, it is used for making tonic water.[14]
In some areas, non-medical use of quinine is regulated. For example, in the الولايات المتحدة and in ألمانيا, quinine is limited to between 83-85 parts per million.[15] In order to achieve a therapeutic dose of quinine from tonic water, a person would have to drink between 6 and 12 litres in a 24-hour period.[16]
In فرنسا, quinine is an ingredient of an apéritif known as Quinquina.
Because of its relatively constant and well-known fluorescence quantum yield, quinine is also used in photochemistry as a common fluorescence standard.
Quinine (and quinidine) are used as the chiral moiety for the ligands used in Sharpless asymmetric dihydroxylation.
Quinine is sometimes added to the recreational drugs cocaine, heroin and others in order to "cut" the product and make more profit.
In كندا, quinine is an ingredient in the carbonated chinotto beverage called Brio.
In the المملكة المتحدة, Scottish company A.G. Barr's uses quinine as an ingredient in the carbonated and caffeinated beverage Irn-Bru.
In إنگلترة, Australia, New Zealand and Egypt, quinine is an ingredient in Schweppes and other Indian tonic waters, at a concentration of 0.4 mg/l.[بحاجة لمصدر]
In Uruguay and الأرجنتين, quinine is an ingredient of a Pepsico Inc. Tonic water named Paso de los Toros.
In South Africa, quinine is an ingredient of a Clifton Instant Drink named Chikree produced by Tiger Food Brands.
انظر أيضاً
- Pharmacology
- Luis Jerónimo Fernández de Cabrera and Jesuit's bark, for the story of its introduction into Europe
- Stockwell JR. Aeromedical considerations of malaria prophylaxis with mefloquine hydrochloride. Aviation, Space, and Environmental Medicine 1982; 3(10):1011-3
المصادر
- ^ أ ب ت Conner, Clifford D. (2005). A People's History of Science: Miners, Midwives, and ‘Low Mechanicks’. New York: Nation Books. pp. 95–96. ISBN 1560257482.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) Also cites Porter, Roy (1998). The Greatest Benefit to Mankind: A Medical History of Humanity. New York: W. W. Norton. pp. 465–466. ISBN 0393046346.{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Woodward R, Doering W (1944). "The Total Synthesis of Quinine". J Am Chem Soc. 66 (849).
- ^ Kaufman, Teodoro S. (2005). "Die Jagd auf Chinin: Etappenerfolge und Gesamtsiege". Angewandte Chemie, Int. Ed. 117 (6): 876–907. doi:10.1002/ange.200400663.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barennes H; et al. (1996). "Efficacy and pharmacokinetics of a new intrarectal quinine formulation in children with Plasmodium falciparum malaria". Brit J Clin Pharmacol. 41 (5): 389. doi:10.1046/j.1365-2125.1996.03246.x.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Barennes, H. (2006). "Safety and efficacy of rectal compared with intramuscular quinine for the early treatment of moderately severe malaria in children: randomised clinical trial". Brit Med J. 332 (7549): 1055–57. doi:10.1136/bmj.332.7549.1055. PMID 16675812.
{{cite journal}}: Unknown parameter|unused_data=ignored (help) - ^ BNF 56: Nocturnal leg cramps Accessed 30/11/2008
- ^ Center for Disease Control (1991). "Treatment with Quinidine Gluconate of Persons with Severe Plasmodium falciparum Infection: Discontinuation of Parenteral Quinine". Morb Mort Weekly Rep. 40 (RR-4): 21–23. Retrieved 2006-05-06.
- ^ Magill A, Panosian C (2005). "Making Antimalarial Agents Available in the United States". New Engl J Med. 353 (4): 335–337. doi:10.1056/NEJMp058167.
- ^ Optically active isomers of quinine and quinidine and their respective biological action Accessed 26/1/2009
- ^ Dannenberg AL (1983). "Use of quinine for self-induced abortion". The Southern Medical Journal. 76 (7): 846–849. PMID 0038-4348.
{{cite journal}}: Check|pmid=value (help) - ^ Department of Clinical Pharmacology, Huddinge University Hospital, Sweden (1994). "The concentration-effect relationship of quinine-induced hearing impairment". Clin Pharmacol Ther. 55 (3): 317–323. PMID 8143397.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ FDA Consumer Magazine (1995 July-August). "FDA Orders Stop to Marketing Of Quinine for Night Leg Cramps". Retrieved 2008-02-15.
{{cite web}}: Check date values in:|date=(help) - ^ United States Food and Drug Administration (December 2006). "FDA Orders Unapproved Quinine Drugs from the Market and Cautions Consumers About Off-Label Use of Quinine to Treat Leg Cramps". Retrieved 2008-02-15.
- ^ Hobhouse, Henry (2004). [Šest rostlin, které změnily svět] Error: {{Lang}}: unrecognized language code: cz (help) (in Czech). Prague: Akademie věd České republiky. p. 59. ISBN 802001179X.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)CS1 maint: unrecognized language (link) - ^ Ballestero, Jimena A. (2005). "Effects of Quinine, Quinidine, and Chloroquine on α9α10 Nicotinic Cholinergic Receptors". Molecular Pharmacology. 68 (3): 822–829. doi:10.1124/mol.105.014431.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Whole Foods Markets: Ingredients: Quinine". Whole Foods Market, IP, L.P. Retrieved 2008-06-21.
للاستزادة
- Wolff RS, Wirtschafter D, Adkinson C (1997). "Ocular quinine toxicity treated with hyperbaric oxygen". Undersea Hyperb Med. 24 (2): 131–4. PMID 9171472. Retrieved 2008-08-13.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
وصلات خارجية
- Articles containing ألمانية-language text
- CS1 errors: unsupported parameter
- CS1 errors: PMID
- Lang and lang-xx template errors
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Articles without EBI source
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- مقالات ذات عبارات بحاجة لمصادر
- Articles with hatnote templates targeting a nonexistent page
- Ethers
- كينين
- كينولينات
- Bitter compounds
- عقاقير مجهضة
- Quinuclidines
- الأدوية الأساسية حسب منظمة الصحة العالمية
