بيسوپرولول
 | |
| البيانات السريرية | |
|---|---|
| الأسماء التجارية | زبـِتا، كونكور |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693024 |
| License data |
|
| فئة السلامة أثناء الحمل |
|
| مسارات الدواء | oral |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية |
|
| بيانات الحركية الدوائية | |
| التوافر الحيوي | >90% |
| ارتباط الپروتين | 30%[1] |
| الأيض | 50% Hepatic |
| Elimination half-life | 10–12 hours[2] |
| المعرفات | |
| |
| رقم CAS | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.108.941 |
| Chemical and physical data | |
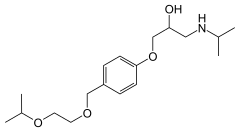
| التركيب | C18H31NO4 |
| الكتلة المولية | 325.443 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
بيسوپرولول Bisoprolol، هو عقار من مجموعة حاصرات بيتا، ويستخدم بصفة أساسية لعلاج الأمراض القلبية الوعائية. أكثر تحديدا، فهو نوع مختار من حاصرات مستقبلات الأدرينالينβ1. وافقت ادارة الغذاء والدواء على الشكل الصيدلاني من عقار بيسوپرولول في شكل أقراص والمقدم من شركة زبـِتا في 31 يوليو 1992. ويتم تصنيع العقار من قبل شركات تيڤا، ميلان، ساندوز، Mutual Pharmaceutical Company.[3]
الاستخدام السريري
Bisoprolol is beneficial in treatment for: high blood pressure (hypertension), reduced blood flow to the heart (cardiac ischemia); preventative treatment before and primary treatment after heart attacks decreasing the chances of recurrence.[4] During hypertension there is an elevated blood pressure, which is what Bisoprolol targets.[5][6] While in cardiac ischemia the drug is used to reduce the activity of the heart muscle and therefore reduce oxygen and nutrient demand, so reduced blood supply can still transport sufficient amounts of oxygen and nutrients. [24][27][30]
Many beta-blockers are now available and in general they are all equally effective. There are, however, differences between them which may affect choice in treating particular diseases or individual patients.
Beta-blockers with a relatively short duration of action have to be given two or three times daily. Many of these are, however, available in modified-release formulations so that administration once daily is adequate for hypertension. For angina twice-daily treatment may sometimes be needed even with a modified-release formulation. Some beta-blockers such as atenolol, bisoprolol, carvedilol, celiprolol, and nadolol have an intrinsically longer duration of action and need to be given only once daily.
آلية العقار
Bisoprolol is cardioprotective because it selectively and competitively blocks catecholamine (adrenalin) stimulation of beta-1 adrenergic receptors (β1 adrenoreceptor) mainly found in the heart muscle cells and heart conduction tissue (cardio specific) but also found in juxtaglomerular cells in the kidney [24]. Normally adrenalin and noradrenalin stimulation of the β1 adrenoreceptor activates a signalling cascade (Gs protein and cAMP) which ultimately lead to increased contractility and increased heart rate of the heart muscle and heart pacemaker respectively [23]. Bisoprolol competitively blocks the activation of this cascade and therefore decreases the adrenergic tone/stimulation of the heart muscle and pacemaker cells. Decreased adrenergic tone shows less contractility of heart muscle and lowered heart rate of heart pacemaker [25][28][29].
These are the favourable factors that are decreased and treat hypertension, heart attacks and ischemia. The decreases in contractility and heart rate are beneficial for hypertension because they reduce blood pressure[5][27] but for preventive measures for heart attacks and cardiac ischemia these decreases in heart rate and contraction decrease the hearts demand for oxygen and nutrients; primary treatment post heart attacks is to prevent recurrence of the infarction [27][30][6].
تحذيرات
Beta-blockers can precipitate asthma and this effect can be dangerous. Beta-blockers should be avoided in patients with a history of asthma or bronchospasm; if there is no alternative, a cardioselective beta-blocker can be used with extreme caution under specialist supervision. Atenolol, bisoprolol, metoprolol, nebivolol, and (to a lesser extent) acebutolol, have less effect on the beta2 (bronchial) receptors and are, therefore, relatively cardioselective, but they are not cardiospecific. They have a lesser effect on airways resistance but are not free of this side effect.
الآثار الجانبية
Overdose of Bisoprolol leads to fatigue, hypotension[27], low blood sugar [29], bronchospasms and bradycardia [27]. Bronchospasms and low blood sugar because at high doses drug can be an antagonist for β2 adrenergic receptors located in lung and in liver. Bronchspasm due to blockage in lungs of β2 receptor and low blood sugar because of decreased stimulation of glycogenolysis and gluconeogenesis in the liver via β2 receptor.[24][26][27]
الأعراض
Bisoprolol (Concor,[7] Zebeta,[8] Concore,[9] Monocor[10]) can be used to treat cardiovascular diseases such as hypertension, coronary heart disease, arrhythmias, ischemic heart diseases and treatment of myocardial infarction after the acute event. Patients with compensated congestive heart failure may be treated with Bisoprolol as a comedication (usually together with an ACE inhibitor, a diuretic and a digitalis-glycosid, if indicated). In patients with congestive heart failure, it reduces the need for and the consumption of oxygen of the heart muscle. It is very important to start with low doses, as bisoprolol reduces also the muscular power of the heart, which is an undesired effect in congestive heart failure.
The drug is also used to treat other conditions, including dysautonomia, anxiety and hyperthyroidism (over active thyroid gland).
Bisoprolol will give a positive result in doping tests.[11]
علم الصيدلة والكيمياء الحيوية
Bisoprolol has both lipid and water soluble properties making it a prime candidate over other β-blockers and even over other β1-blockers, being water soluble it will have decreased incidence of central nervous system side effects (inability to diffuse into brain) compared to purely lipophilic compounds [25][28]. Bisoprolol has an approximate half-life of 10-12 hours and when ingested has nearly complete absorption into the blood stream[28][29]. The high absorption is indicative of high bioavailability (approx. 90%)[28][29]. When being eliminated, the body evenly distributes it (50-50) between kidney excretion and liver biotransformation (then excreted) [25][28][29]. These factors make it a convenient once/day dosage when it’s being administered. [28][29]
β1 Selectivity
Bisoprolol beta 1-selectivity is especially important in comparison to other non-selective beta blockers. The effects of the drug are limited to areas containing β1 adrenoreceptors which is mainly the heart and a little bit of the kidney [25][28]. Bisoprolol minimizes the side effects that might occur from administration of a non-specific beta blocker where blockage of the other adrenoreceptors (β2, β3, α1, α2) occurs. The other receptors elicit a variety of responses in the body and blockage of them could cause a wide range of reactions; but β1 adrenoreceptors are cardio specific for the most part, making Bisoprolol ideal for treatment of cardiac events [25][29]. Bisoprolol has a higher degree of β1-selectivity compared to other β1-selective β-blockers such as atenolol, metoprolol and betaxolol.[12][13][14][15][16][17][18][19][20][21] However Nebivolol is approximately 3.5 times more β1-selective.[22][23]
Antihypertensive effect
Bisoprolol has a stronger antihypertensive effect than propranolol.[12]
Cardioprotection
Bisoprolol in animal models has been shown to be cardioprotective.[12]
Renin-angiotensin system
Bisoprolol inhibits renin secretion by about 65% and tachycardia by about 35%.[12]
Pharmacology of side effects
In animal testing bisoprolol compared to propranolol has shown less sedative effects and only slightly reduced glucose tolerance.[24]
المصادر
- ^ Bühring KU, Sailer H, Faro HP, Leopold G, Pabst J, Garbe A (1986). "Pharmacokinetics and metabolism of bisoprolol-14C in three animal species and in humans". J. Cardiovasc. Pharmacol. 8 Suppl 11: S21–8. PMID 2439794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leopold G (1986). "Balanced pharmacokinetics and metabolism of bisoprolol". J. Cardiovasc. Pharmacol. 8 Suppl 11: S16–20. PMID 2439789.
- ^ http://www.drugs.com/pro/bisoprolol.html
- ^ Rosenberg, Jens, and Finn Gustafsson. "Bisoprolol for Congestive Heart Failure." Expert Opinion on Pharmacotherapy 9.2 (2008): 293-300. Print
- ^ أ ب Amabile, G. And Serradimigni, A. "Comparison of bisoprolol with nifedipine for treatment of essential hypertension in the elderly: Comparative double-blind trial” European Heart Journal 8 (1987): 65-69. Web. 5 Feb. 2011
- ^ أ ب Thadani, Udho. "Beta Blockers in Hypertension." The American Journal of Cardiology 52.9 (1983): D10-15. Web. 6 Feb. 2011
- ^ Bisoprolol at Merck Serono
- ^ "Prescription Drugs: Zebeta". Physicians' Desktop Reference. Retrieved 2007-12-23.
- ^ "Pharmaceuticals". Merck Philippines. Archived from the original on 2007-12-22. Retrieved 2008-01-01.
- ^ "Products: Monocor". Biovail Corporation. Retrieved 2007-12-23.
- ^ Ratiopharm packing slip, dated 03.08.2006
- ^ أ ب ت ث Harting J, Becker KH, Bergmann R; et al. (1986). "Pharmacodynamic profile of the selective beta 1-adrenoceptor antagonist bisoprolol". Arzneimittelforschung. 36 (2): 200–8. PMID 2870720.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Haeusler G, Schliep HJ, Schelling P; et al. (1986). "High beta 1-selectivity and favourable pharmacokinetics as the outstanding properties of bisoprolol". J. Cardiovasc. Pharmacol. 8 Suppl 11: S2–15. PMID 2439793.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Kaumann AJ, Lemoine H (1985). "Direct labelling of myocardial beta 1-adrenoceptors. Comparison of binding affinity of 3H-(-)-bisoprolol with its blocking potency". Naunyn Schmiedebergs Arch. Pharmacol. 331 (1): 27–39. PMID 2866449.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Klockow M, Greiner HE, Haase A, Schmitges CJ, Seyfried C (1986). "Studies on the receptor profile of bisoprolol". Arzneimittelforschung. 36 (2): 197–200. PMID 2870719.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Manalan AS, Besch HR, Watanabe AM (1981). "Characterization of [3H](+/-)carazolol binding to beta-adrenergic receptors. Application to study of beta-adrenergic receptor subtypes in canine ventricular myocardium and lung". Circ. Res. 49 (2): 326–36. PMID 6113900.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schliep HJ, Schulze E, Harting J, Haeusler G (1986). "Antagonistic effects of bisoprolol on several beta-adrenoceptor-mediated actions in anaesthetized cats". Eur. J. Pharmacol. 123 (2): 253–61. doi:10.1016/0014-2999(86)90666-7. PMID 3011461.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schliep HJ, Harting J (1984). "Beta 1-selectivity of bisoprolol, a new beta-adrenoceptor antagonist, in anesthetized dogs and guinea pigs". J. Cardiovasc. Pharmacol. 6 (6): 1156–60. doi:10.1097/00005344-198406060-00024. PMID 6084774.
- ^ Schnabel P, Maack C, Mies F, Tyroller S, Scheer A, Böhm M (2000). "Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors". J. Cardiovasc. Pharmacol. 36 (4): 466–71. PMID 11026647.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Smith C, Teitler M (1999). "Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors" (PDF). Cardiovasc Drugs Ther. 13 (2): 123–6. doi:10.1023/A:1007784109255. PMID 10372227.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wellstein A, Palm D, Belz GG (1986). "Affinity and selectivity of beta-adrenoceptor antagonists in vitro". J. Cardiovasc. Pharmacol. 8 Suppl 11: S36–40. PMID 2439796.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bundkirchen A, Brixius K, Bölck B, Nguyen Q, Schwinger RH (2003). "Beta 1-adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies". Eur. J. Pharmacol. 460 (1): 19–26. doi:10.1016/S0014-2999(02)02875-3. PMID 12535855.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nuttall SL, Routledge HC, Kendall MJ (2003). "A comparison of the beta1-selectivity of three beta1-selective beta-blockers". J Clin Pharm Ther. 28 (3): 179–86. doi:10.1046/j.1365-2710.2003.00477.x. PMID 12795776.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lettenbaur H. EMD 33 512 (Bisoprolol): Prüfung der Wirkung auf die Serumglukosekonzentration an Ratten im Vergleich zu Propranolol. Merck KGaA, Darmstadt, 1979.
23. Bristow, MR., Hershberger, RE., Port, JD., Minobe, W., Rasmussen, R. “Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium” Molecular Pharamcology 35 (1989): 295-203. Web. 5 Feb. 2011.
24. CIBIS Investigators and Committees. "A Randomized Trial of Beta-blockade in Heart Failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS)." Circulation 90 (1994): 1765-773. Web. 4 Feb. 2011.
25. Haeusler, G., HJ Schliep ... Ect. All. "High Beta 1-selectivity and Favourable Pharmacokinetics as the Outstanding Properties of Bisoprolol." Journal of Cardiovascular Pharmacology 2-15 8 (1986). Web. 5 Feb. 2011.
26. Hauck, R. W., Ch Schulz, H. P. Emslander, and *M Bohm. "Pharmacological Actions of the Selective and Non-selective Beta-adrenoceptor Antagonists Celiprolol, Bisoprolol and Propranolol on Human Bronchi." British Journal of Pharmacology (1994): 1043-049. Web. 5 Feb. 2011.
27. Konishi, M., and G. Haraguchi ... Ect. All. "Comparative Effects of Carvedilol vs Bisoprolol for Severe Congestive Heart Failure." Circulation 6 (2010): 1127-134. Web. 6 Feb. 2011.
28. Leopold, G., W. Ungethcm ... Etc. All. "Basic Pharmacokinetics of Bisoprolol, a New Highly Beta 1-selective Adrenoceptor Antagonist." Journal of Clinical Pharmacology 26 (1986): 616-21. Web. 7 Feb. 2011
29. Leopold, G., W. Ungethcm ... Etc. All. "Pharmacodynamic Profile of Bisoprolol, a New Beta1-selective Adrenoceptor Antagonist." British Journal of Clinical Pharmacology 22 (1986): 293-300. Web. 6 Feb. 2011.
30. Poolewilson, P. "The Cardiac Insufficiency Bisoprolol Study II." The Lancet 353.9161 (1999): 1360. Print.
وصلات خارجية
- CS1 errors: unsupported parameter
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed DrugBank identifier
- ECHA InfoCard ID from Wikidata
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Infobox drug articles with non-default infobox title
- Drugboxes which contain changes to verified fields
- حاصرات بيتا
- إثيرات
- إثيرات الفنول
- كحولات