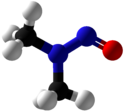
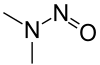
ن-نيتروزو ثنائي المثيلامين
| |||
|
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك المفضل
N,N-Dimethylnitrous amide | |||
| المُعرِّفات | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.500 | ||
| رقم EC |
| ||
| KEGG | |||
| عناوين مواضيع طبية MeSH | |||
PubChem CID
|
|||
| رقم RTECS |
| ||
| UNII | |||
| UN number | 3382 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| الخصائص | |||
| الصيغة الجزيئية | C2H6N2O | ||
| كتلة مولية | 74.08 g mol-1 | ||
| المظهر | Yellow oil[1] | ||
| الرائحة | faint, characteristic[1] | ||
| الكثافة | 1.005 g/mL | ||
| نقطة الغليان | |||
| قابلية الذوبان في الماء | 290 mg/ml (at 20 °C) | ||
| log P | −0.496 | ||
| ضغط البخار | 700 Pa (at 20 °C) | ||
| معامل الانكسار (nD) | 1.437 | ||
| الكيمياء الحرارية | |||
| الانتالبية المعيارية للاحتراق ΔcH |
1.65 MJ/mol | ||
| المخاطر | |||
| خطر رئيسي | Known carcinogen[1], extremely toxic | ||
| ن.م.ع. مخطط تصويري |   
| ||
| ن.م.ع. كلمة الاشارة | DANGER | ||
| H301, H330, H350, H372, H411 | |||
| P260, P273, P284, P301+P310, P310 | |||
| NFPA 704 (معيـَّن النار) | |||
| نقطة الوميض | 61.0 °C (141.8 °F; 334.1 K) | ||
| الجرعة أو التركيز القاتل (LD, LC): | |||
LD50 (الجرعة الوسطى)
|
37.0 mg/kg (oral, rat) | ||
| حدود التعرض الصحية بالولايات المتحدة (NIOSH): | |||
PEL (المسموح)
|
OSHA-Regulated Carcinogen[1] | ||
REL (الموصى به)
|
Ca[1] | ||
IDLH (خطر عاجل)
|
Ca [N.D.][1] | ||
| مركبات ذا علاقة | |||
مركـّبات ذات علاقة
|
|||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of N-nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in lab animals.[2]
التواجد
مياه الشرب
Of more general concern, NDMA can be produced by water treatment by chlorination or chloramination. The question is the level at which it is produced. In the U.S. state of California, the allowable level is 10 nanograms/liter. The Canadian province of Ontario set the standard at 9 ng/L. The potential problem is greater for recycled water that can contain dimethylamine.[3] Further, NDMA can form or be leached during treatment of water by anion exchange resins.[4]
NDMA's contamination of drinking water is of particular concern due to the minute concentrations at which it is harmful, the difficulty in detecting it at these concentrations, and to the difficulty in removing it from drinking water. It does not readily biodegrade, adsorb, or volatilize. As such, it cannot be removed by activated carbon and travels easily through soils. Relatively high levels of UV radiation in the 200 to 260 nm range breaks the N–N bond and can thus be used to degrade NDMA. Additionally, reverse osmosis removes approximately 50% of NDMA.[5]
اللحم المقدد
NDMA is found at low levels in numerous items of human consumption, including cured meat, fish, beer, and tobacco smoke.[4][6] The pathway[محل شك] for NDMA formation involves nitrous acid produced from sodium nitrite, which is used to cure meat.[vague] NDMA arises[when?]قالب:How? by the combination of nitrous acid and dimethylamine derived from the degradation of dietary protein in the lower gut.[vague]
وقود الصواريخ
Unsymmetrical dimethylhydrazine, a rocket fuel, is a highly effective precursor to NDMA:
- (CH3)2NNH2 + 2 O → (CH3)2NNO + H2O
Groundwater near rocket launch sites often has high levels of NDMA.[5]
التنظيم
التركيب الكيميائي
The C2N2O core of NDMA is planar, as established by X-ray crystallography. The central nitrogen is bound to two methyl groups and the NO group with bond angles of 120°. The N-N and N-O distances are 1.32 and 1.26 Å, respectively.[7]
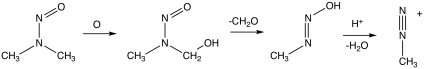
NDMA forms from a variety of dimethylamine-containing compounds, e.g. hydrolysis of dimethylformamide. Dimethylamine is susceptible to oxidation to unsymmetrical dimethylhydrazine, which air-oxidizes to NDMA.[8]
In the laboratory, NDMA can be synthesised by the reaction of nitrous acid with dimethylamine:
- HONO + (CH3)2NH → (CH3)2NNO + H2O
The mechanism of its carcinogenicity involves metabolic activation steps resulting in the formation of methyl diazonium, an alkylating agent.[2]
كمادة سامة
Several incidents in which NDMA was used to intentionally poison another person have garnered media attention. In 1978, a teacher in Ulm, Germany, was sentenced to life in prison for trying to murder his wife by poisoning jam with NDMA and feeding it to her. Both the wife and the teacher later died from liver failure.[9][10] Also in 1978, Steven Roy Harper spiked lemonade with NDMA at the Johnson family home in Omaha, Nebraska. The incident resulted in the deaths of 30-year-old Duane Johnson and 11-month-old Chad Shelton. For his crime, Harper was sentenced to death, but committed suicide in prison before his execution could be carried out.[11][استشهاد ناقص]
In the 2013 Fudan poisoning case, Huang Yang, a postgraduate medical student at Fudan University, was the victim of a poisoning in Shanghai, China. Huang was poisoned by his roommate Lin Senhao, who had placed NDMA into the water cooler in their dormitory. Lin claimed that he only did this as an April Fool's joke. He received a death sentence, and was executed in 2015.[12] In 2018, NDMA was used in an attempted poisoning at Queen's University in Kingston, Canada.[13]
في الأدوية
In 2018, and then again in late 2019, various brands of valsartan were recalled because of contamination with N-nitrosodimethylamine.[14] In 2019, ranitidine was recalled across the world due to contamination with NDMA.[15] In December 2019, the FDA began testing samples of the diabetes drug metformin for the carcinogen N-nitrosodimethylamine (NDMA). The FDA's announcement followed a recall of three versions of metformin in Singapore, and the European Medicines Agency's request that manufacturers test for NDMA.[16][17]
In September 2019, the carcinogen N-nitrosodimethylamine (NDMA) was discovered in ranitidine products from a number of manufacturers, resulting in recalls.[18][19][20][21][22][23] In April 2020, it was withdrawn from the United States market and suspended in the European Union due to these concerns.[20][24][25]
في 9 أكتوبر 2020، سحبت إدارة الغذاء والدواء الأمريكية دواء متوفورمن هيدروكلوريد المستخدم في علاج مرض السكري لاحتوائه على نسبة عالية من مواد مسببة للسرطان. قامت الشركة المنتجة للدواء، ماركانس فارما الهندية، بسحب أقراص متوفورمن هيدروكلوريد ممتدة المفعول لاحتوائها على مستويات مرتفعة من مادة ن-نيتروزو ثنائي المثيلامين NDMA، التي تعتبر مادة مسرطنة محتملة لدى البشر"، والتي تتجاوز الجرعة اليومية المقبولة، 96 نانوگرام/يومياً"، وذلج بحسب منشور السحب الصادر عن إدارة الغذاء والدواء.[26]
تستخدم أقراص متفورمن لعلاج سكري النمط الثاني لخفض مستويات گلوكوز الدم. السحب ساري أقراص متوفورمن جرعة 500 ملگ و750 ملگ، التي تباع تحت الاسم التجاري تايم-كيپ لابس Time-Cap Labs.
جاء هذا السحب كتكملة لعملية سحب سابقة لنفس المنتج في يوليو 2020. هذا المنتج من متوفورمن واحداً من ضمن العديد من منتجات المتوفورمن التي اكتشف أنها تحتوي على NDMA في 2019. أصدرت سبع شركات أدوية أخرى عمليات سحب لأقراص ميفورمين هيدروكلوريد ممتد المفعول بسبب محتوياتها المسببة للسرطان.
المصادر
- ^ أ ب ت ث ج ح NIOSH Pocket Guide to Chemical Hazards 0461
- ^ أ ب ت Tricker, A.R.; Preussmann, R. (1991). "Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential". Mutation Research/Genetic Toxicology. 259 (3–4): 277–289. doi:10.1016/0165-1218(91)90123-4. PMID 2017213.
- ^ "Sources and Fate of Nitrosodimethylamine and Its Precursors in Municipal Wastewater Treatment Plants". Water Environment Research. 77 (1, Emerging Micropollutants in Treatment Systems (Jan.–Feb. 2005)): 32–39. 2005. doi:10.2175/106143005X41591. JSTOR 25045835. PMID 15765933.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ أ ب Najm, I.; Trussell, R. R. (2001). "NDMA Formation in Water and Wastewater". Journal American Water Works Association. 93 (2): 92–99. doi:10.1002/j.1551-8833.2001.tb09129.x. ISSN 0003-150X.[dead link]
- ^ أ ب Mitch, W. A.; Sharp, J. O.; Trussell, R. R.; Valentine, R. L.; Alvarez-Cohen, L.; Sedlak, D. L. (2003). "N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review". Environmental Engineering Science. 20 (5): 389–404. CiteSeerX 10.1.1.184.204. doi:10.1089/109287503768335896.
- ^ Hecht, Stephen S. (1998). "Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines†". Chemical Research in Toxicology. 11 (6): 559–603. doi:10.1021/tx980005y. PMID 9625726.
- ^ Krebs, Bernt; Mandt, Jürgen (1975). "Kristallstruktur des N-Nitrosodimethylamins". Chemische Berichte. 108 (4): 1130–1137. doi:10.1002/cber.19751080419.
- ^ Mitch, William A.; Sedlak, David L. (2002). "Formation of N-Nitrosodimethylamine (NDMA) from Dimethylamine during Chlorination". Environmental Science & Technology. 36 (4): 588–595. Bibcode:2002EnST...36..588M. doi:10.1021/es010684q. PMID 11878371.
- ^ Ein teuflischer Plan: Tod aus dem Marmeladeglas (in German).
- ^ Karsten Strey: "Die Welt der Gifte", Lehmanns, 2. Edition p. 193 (in German).
- ^ Roueche, Betron (January 25, 1982). "Annals of Medicine – The Prognosis for this Patient is Horrible". The New Yorker: 57–71.[استشهاد ناقص]
- ^ "15 days log in hospital". Archived from the original on 2014-01-09. Retrieved 2013-04-30.
- ^ https://www.thewhig.com/news/local-news/man-admits-poisoning-fellow-researcher-in-kingston?
- ^ EMA Staff (September 17, 2018). "EMA reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity: some being recalled across the EU". European Medicines Agency (in الإنجليزية). Retrieved December 3, 2019.
- ^ BBC Staff (September 29, 2019). "Sale of heartburn drug suspended over cancer fears". BBC News (in الإنجليزية البريطانية). Retrieved December 3, 2019.
- ^ Lauerman, John (December 4, 2019). "Diabetes Drugs Latest to Be Targeted for Carcinogen Scrutiny". Bloomberg Business. Retrieved January 5, 2020.
{{cite journal}}: CS1 maint: url-status (link) - ^ Koenig, D. (December 6, 2019). "FDA Investigating Metformin for Possible Carcinogen". Medscape. Retrieved December 14, 2019.
- ^ "Health Canada assessing NDMA in ranitidine". Health Canada. 13 September 2019. Archived from the original on 26 September 2019. Retrieved 26 September 2019.
- ^ "Statement alerting patients and health care professionals of NDMA found in samples of ranitidine". U.S. Food and Drug Administration (FDA). 13 September 2019. Archived from the original on 26 September 2019. Retrieved 26 September 2019.
 هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ أ ب "Questions and Answers: NDMA impurities in ranitidine (commonly known as Zantac)". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 24 October 2019. Retrieved 23 October 2019.
 هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ "EMA to provide guidance on avoiding nitrosamines in human medicines". European Medicines Agency (EMA) (Press release). 13 September 2019. Retrieved 19 September 2019.
 هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ "EMA to review ranitidine medicines following detection of NDMA". European Medicines Agency (EMA) (Press release). 13 September 2019. Retrieved 19 September 2019.
- ^ "FDA updates and press announcements on NDMA in Zantac (ranitidine)". U.S. Food and Drug Administration (FDA). 28 October 2019. Archived from the original on 29 October 2019. Retrieved 28 October 2019.
FDA observed the testing method used by a third-party laboratory uses higher temperatures. The higher temperatures generated very high levels of NDMA from ranitidine products because of the test procedure. FDA published the method for testing angiotensin II receptor blockers (ARBs) for nitrosamine impurities. That method is not suitable for testing ranitidine because heating the sample generates NDMA.
FDA recommends using an LC-HRMS testing protocol to test samples of ranitidine. FDA's LC-HRMS testing method does not use elevated temperatures and has shown the presence of much lower levels of NDMA in ranitidine medicines than reported by the third-party laboratory. International regulators using similar LC-MS testing methods have also shown the presence of low levels of NDMA in ranitidine samples. هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ "FDA Requests Removal of All Ranitidine Products (Zantac) from the Market". U.S. Food and Drug Administration (FDA) (Press release). 1 April 2020. Retrieved 1 April 2020.
 هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ "Suspension of ranitidine medicines in the EU". European Medicines Agency (Press release). 30 April 2020. Retrieved 2 June 2020.
 هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
هذا المقال يضم نصاً من هذا المصدر، الذي هو مشاع.
- ^ "A diabetes drug has been recalled because it contains high levels of cancer-causing agent". سي إن إن. 2020-10-09. Retrieved 2020-10-12.
وصلات خارجية
- Nitrosodimethylamine (NDMA) Information
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Method Development for the Determination of N-Nitrosodimethylamine (NDMA) in Drinking Water
- SFPUC NDMA White Paper
- Public Health Statement for n-Nitrosodimethylamine
- Toxicological Profile for n-Nitrosodimethylamine CAS# 62-75-9
- CS1 errors: deprecated parameters
- Articles with dead external links from February 2018
- Articles with incomplete citations from January 2020
- All articles with incomplete citations
- CS1 الإنجليزية البريطانية-language sources (en-gb)
- CS1 maint: url-status
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed KEGG identifier
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Short description is different from Wikidata
- مقالات ذات عبارات محل شك
- All Wikipedia articles needing clarification
- Wikipedia articles needing clarification from July 2020
- Vague or ambiguous time from July 2020
- Articles with hatnote templates targeting a nonexistent page
- Nitrosamines
- IARC Group 2A carcinogens
- ذيفانات الكبد