قوة بين جزيئية
التفاعلات غير الارتباطية intermolecular force هي التفاعلات التى تتم عن طريق القوى الكهرساكنة أو قوى فان دير فال بين الذرات غير المرتبطة معا.
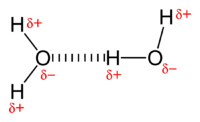
روابط الهيدروجين
A hydrogen bond is the attraction between the lone pair of an electronegative atom and a hydrogen atom that is bonded to either nitrogen, oxygen, or fluorine.[1] The hydrogen bond is often described as a strong electrostatic dipole-dipole interaction. However, it also has some features of covalent bonding: it is directional, stronger than a van der Waals force interaction, produces interatomic distances shorter than the sum of van der Waals radius, and usually involves a limited number of interaction partners, which can be interpreted as a kind of valence.
Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides, which have no hydrogen bonds. Intramolecular hydrogen oxygen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.[2]
تآثر ثنائيي القطب وما شابه
Regular dipole
Dipole–dipole interactions are electrostatic interactions between molecules which have permanent dipole(s).These interactions tend to align the molecules to increase attraction (reducing potential energy). An example of a dipole–dipole interaction can be seen in hydrogen chloride (HCl): the positive end of a polar molecule will attract the negative end of the other molecule and influence its position. Polar molecules have a net attraction between them. Examples of polar molecules include hydrogen chloride (HCl) and chloroform (CHCl3).
Often molecules contain dipolar groups, but have no overall dipole moment. This occurs if there is symmetry within the molecule that causes the dipoles to cancel each other out. This occurs in molecules such as tetrachloromethane and carbon dioxide. The dipole-dipole interaction between two individual atoms is usually zero, since atoms rarely carry a permanent dipole.These forces are discussed further in the section about the Keesom interaction, below.
Ion–dipole and ion-induced dipole forces
Ion-dipole and ion-induced dipole forces are similar to dipole-dipole and dipole-induced dipole interactions but involve ions, instead of only polar and non-polar molecules. Ion-dipole and ion-induced dipole forces are stronger than dipole-dipole interactions because the charge of any ion is much greater than the charge of a dipole moment. Ion-dipole bonding is stronger than hydrogen bonding.[3]
An ion–dipole force consists of an ion and a polar molecule interacting. They align so that the positive and negative groups are next to one another, allowing maximum attraction.
An ion-induced dipole force consists of an ion and a non-polar molecule interacting. Like a dipole-induced dipole force, the charge of the ion causes distortion of the electron cloud on the non-polar molecule.[4]
قوى ڤان در ڤالس
The van der Waals forces arise from interaction between uncharged atoms or molecules, leading not only to such phenomena as the cohesion of condensed phases and physical adsorption of gases, but also to a universal force of attraction between macroscopic bodies.[5]
تفاعل كيسوم (بين ثنائيات الأقطاب الدائمة)
الشدة النسبية للقوى
| نوع الرابطة | Dissociation energy (kcal)[6],[7] |
|---|---|
| Covalent | 400 |
| Hydrogen bonds | 12-16 |
| Dipole-dipole | 0.5 - 2 |
| London (van der Waals) Forces | <1 |
Note: this comparison is only approximate- the actual relative strengths will vary depending on the molecules involved.
انظر أيضاً
المصادر
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hydrogen bond".
- ^ Lindh, Ulf (2013), "Biological functions of the elements", in Selinus, Olle, Essentials of Medical Geology (Revised ed.), Dordrecht: Springer, pp. 129–177, ISBN 978-94-007-4374-8, https://doi.org/10.1007/978-94-007-4375-5_7, retrieved on 18 January 2018
- ^ Tro, Nivaldo (2011). Chemistry: A Molecular Approach. United States: Pearson Education Inc. p. 466. ISBN 978-0-321-65178-5.
- ^ Blaber, Michael (1996). Intermolecular Forces. mikeblaber.org
- ^ Leite, F. L.; Bueno, C. C.; Da Róz, A. L.; Ziemath, E. C.; Oliveira, O. N. (2012). "Theoretical Models for Surface Forces and Adhesion and Their Measurement Using Atomic Force Microscopy". International Journal of Molecular Sciences. 13 (12): 12773. doi:10.3390/ijms131012773.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Volland, Dr. Walt. ""Intermolecular" Forces". Retrieved 2009-09-20.
- ^ Organic Chemistry: Structure and Reactivity by Seyhan Ege, pp30-33, 67
وصلات خارجية
- Software for calculation of intermolecular forces
- Quantum 3.2
- SAPT: An ab initio quantumchemical package.