قيمة الحمض
قيمة الحمض Acid value هي عدد ميليغرامات من هيدروكسيد البوتاسيوم KOH اللازمة لتعديل الحموض الحرة في ١غ من الحمض الدسم او المادة المطلوب تحديد قرينة الحمض لها.
The acid number is used to quantify the acidity of a substance e.g. biodiesel. It is the quantity of base, expressed in milligrams of potassium hydroxide, that is required to neutralize the acidic constituents in 1 g of sample.
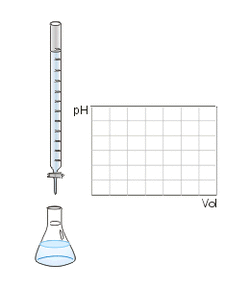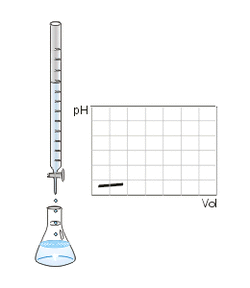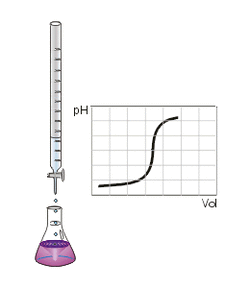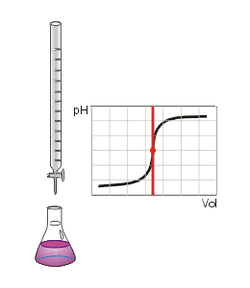
Veq is the volume of titrant (ml) consumed by the crude oil sample and 1 ml of spiking solution at the equivalent point, beq is the volume of titrant (ml) consumed by 1 ml of spiking solution at the equivalent point, and 56.1 g/mol is the molecular weight of KOH. WOil is the mass of the sample in grams.
The molar concentration of titrant (N) is calculated as such:
In which WKHP is the mass (g) of KHP in 50 ml of KHP standard solution, Veq is the volume of titrant (ml) consumed by 50 ml KHP standard solution at the equivalent point, and 204.23 g/mol is the molecular weight of KHP.
انظر أيضاً
المراجع