القشرة الحزامية الأمامية
| المخ: القشرة الحزامية الأمامية | ||
|---|---|---|
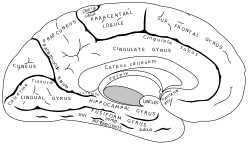
| Medial surface of left cerebral hemisphere. | ||
| NeuroNames | hier-143 | |
القشرة الحزامية الأمامية القشرة الحزامية (ACC) الجزء الأمامى من القشرة الحزامية, التى تشبه "القلادة" تحيط ب corpus callosum , الحزمة الليفية التى تجعل الإشارات العصبية بين اليمين واليسار النصفين الكرويين المخيين للمخ متتابعة.
وهى تشمل كلا من بطني ظهري cingulate cortex , ويبدو أنها تلعب دورا في طائفة واسعة من الوظائف الذاتية , مثل تنظيم ضغط الدم و ضربات القلب , بالإضافة الى يعقل وظائف الإدراكي , مثل مكافأة تحسبا ، وصنع القرار التعاطف و العاطفة.
الوظائف
القشرة الحزامية الأمامية يمكن تقسيمها تشريحيا حسب الوظيفة الى intocutive exe (أمامى), تقييمي (خلفى), (ظهرى), أيضا عاطفى (بطنيالإدراكي]) مكونات.[1] القشرة الحزامية الأمامية تتصل مع الجزء الأمامي من قشرة الدماغ و قشرة جدارية وكذلك الجهاز الحركى و نظام مجالات العين الأمامية .[2] مما يجعلها محطة مركزية للمعالجة من أعلى إلى أسفل من أسفل إلى أعلى ومنبهات وإسناد المراقبة المناسبة إلى مناطق أخرى في الدماغ. ويبدو أن القشرة الحزامية الأمامية المعنية وخاصة عندما تكون هناك حاجة إلى بذل جهد لتنفيذ مثل هذه المهمة في وقت مبكر من حل المشاكل والتعلم.[3] Many دراسات أوجدت وظائف مثل اكتشاف الأخطاء ، تحسبا من المهام و الحافز ، وتعديل ردود الفعل العصبية إلى القشرة الحزامية الأمامية .[1][2][4] ردود فعل القشرة الحزامية الأمامية في Stroop task التجارب (المصممة لقياس التقيد لمتابعة مسارات صنع القرار) لا تزال مرتفعة نسبيا في نمطية مواضيع الإنسان [5], كما البديل- عفوية - يتم التضحية بها. وهي مهمة لإعادة العفوية التي تنتج اصلا, رواية الردود على النقطة المنتجة جامدة ، ونتائج الردود النمطية في تناقص الاستجابة القشرة الحزامية الأمامية .[6] في حين أن معظم الأبحاث الممولة تتركز مهمتها على التركيز على الحد من -- وكثيرا ما يتم تشخيصه ذاتيا حسب اضطراب نقص الانتباه وفرط النشاط (ADHD) - القردة التي تستخدم في الأبحاث الأخيرة قد كشفت عن أن تزايد نشاط القشرة الحزامية الأمامية (وعادة ما ترتبط بانخفاض إستخدام [الدوبامين]] ) يقلل من القدرة على تعلم كيفية استعمال المنبهات البصرية اللازمة لتوقع المكافآت .[7] القشرة الحزامية الأمامية تحتوى على خلايا تعرف بالعصبونات المغزلية عصبون مغزلى , كما تم العثور في القشرة الحزامية الأمامية و قشرة frontoinsular من البشر وغيرهم[[Hominidae | غادر] (القردة العليا)وكذلك في الحيتان الحدباء ، والحوت القاتل وحوت العنبر.
Tasks
A typical task that activates the ACC involves eliciting some form of conflict within the participant that can potentially result in an error. One such task is called the Eriksen flanker task and simply consists of an arrow pointing to the left or right, which is flanked by two distractor arrows creating either compatible (<<<<<) or incompatible (>><>>) trials.[8] Another very common conflict inducing stimulus is the Stroop task (Pardo et al., 1990).
The classic Stroop task involves naming the color ink of words that are either congruent (RED written in red) or incongruent (RED written in blue). Conflict occurs because people’s reading abilities interfere with their attempt to correctly name the word’s ink color. A variation of this task is the Counting-Stroop during which people count either neutral stimuli (‘dog’ presented four times) or interfering stimuli (‘three’ presented four times) by pressing a button.
Another version of the Stroop task named the Emotional Counting Stroop is identical to the Counting Stroop test, except that it also uses segmented or repeated emotional words such as "murder", during the interference part of the task. Using different forms of conflict induction allows researchers to differentiate between the many functions of the ACC.
It is worth noting that all these contrasts involve a subtraction between a more difficult type of trial and a less difficult one. There are reasons to think that ACC responses reflect this difficulty (e.g. increased neural activity associated with signaling more blood flow to other brain regions), rather than cognitive conflict per se .[9] If so, the ACC response is not the neural signature of a brain region that processes the conflict, it is the neural signature of a brain region that is correlated with conflict processing somewhere else.
Evidence from electrical studies
Evidence for the role of the ACC as having an error detection function comes from consistent observations of error related negativity (ERN) uniquely generated within the ACC upon error occurrences.[1][10][11][12] A distinction has been made between an ERP following incorrect responses (response ERN) and a signal after subjects receive feedback after erroneous responses (feedback ERN).
It is worth noting at this point that no-one has clearly demonstrated that the ERN comes from the ACC. Source localization of ERP components is a notoriously messy business. The ERN could come from any number of brain regions, perhaps several. In light of this, it is striking that patients with lateral PFC damage (not medial) showed reduced ERNs.[13]
Reinforcement learning ERN theory poses that there is a mismatch between actual response execution and appropriate response execution, which results in an ERN discharge.[1][11] Furthermore, this theory predicts that when the ACC receives conflicting input from control areas in the brain, it determines and allocates which area should be given control over the motor system. Varying levels of dopamine are believed to influence the optimization of this filter system by providing expectations about the outcomes of an event.
The ERN then, serves as a beacon to highlight the violation of an expectation.[12] Research on the occurrence of the feedback ERN shows evidence that this potential has larger amplitudes when violations of expectancy are large. In other words, if an event is not likely to happen the feedback ERN will be larger if no error is detected. Other studies have examined if the ERN is elicited by varying the cost of an error and the evaluation of a response.[11]
In these trials, feedback is given about whether the participant has gained or lost money after a response. Amplitudes of ERN responses with small gains and small losses were similar. No ERN was elicited for any losses as opposed to an ERN for no wins even though both outcomes are the same. The finding in this paradigm suggests that monitoring for wins and losses is based on the relative expected gains and losses. If you get a different outcome than expected, the ERN will be larger than for expected outcomes. ERN studies have also localized specific functions of the ACC.[12]
The rostral ACC seems to be active after an error commission indicating an error response function, whereas the dorsal ACC is active after both an error and feedback suggesting a more evaluative function (for fMRI evidence see also [14][15][16]). This evaluation is emotional in nature and highlights the amount of distress associated with a certain error.[1] Summarizing the evidence found by ERN studies it appears to be the case that ACC receives information about a stimulus, selects an appropriate response, monitors the action, and adapts behavior if there is a violation of expectancy.[12]
The range of functions attributed to the ACC has been synthesized from many fMRI studies. Some theories focus strictly on the error detection properties of the ACC, while others incorporate conflict monitoring, emotional effects, and reward-based learning. None of the current theories can fully explain the complete picture of the ACC, but each contributes to a piece of the puzzle. Some of the main theories will be discussed in this section.
Error detection theory
The most basic form of ACC theory states that the ACC is involved with error detection.[1] Evidence for this theory has been derived from studies involving a Stroop task.[2] However, ACC activation is also active during correct response and this has been shown using a letter task whereby participants had to respond to the letter X after an A was presented and ignore all other letter combinations with some letters being more competitive than others.[17] They found that for more competitive stimuli ACC activation was greater. This study highlights the important notion that the ACC is not merely involved with detecting an error, but actually evaluates the degree of conflict.
Conflict monitoring theory
This theory poses that the ACC’s primary function is the monitoring of conflict. In the example of the Eriksen flanker task, incompatible trials produce the most conflict and therefore the most activation by the ACC. Evidence for this theory has been demonstrated.[8] Upon detection of a conflict the ACC then provides cues to other areas in the brain to cope with the conflicting control systems. One weakness of this theory is that it cannot explain some evidence obtained by electrical studies[4][11][12] that demonstrate the effects of giving feedback after responses because the theory describes the ACC as strictly monitoring conflict, not as having evaluative properties.
Evidence against error detection and conflict monitoring theory
Several recent studies, particularly those examining task performance related to error and conflict processes in patients with ACC damage, cast doubt on the necessity of this region for these functions.
Baird et al.[18] propose that their data "may imply that the ACC does not have a central role in cognition"
Nachev et al.[19] state that "The highly influential notion of conflict monitoring by the anterior cingulate has been called into question by monkey single-cell neurophysiology and lesion studies in monkeys and humans."
The comprehensive Critchley review[9] states that "The cognitive consequences of anterior cingulate lesions remain rather equivocal, with a number of case reports of intact general neuropsychological and executive function in the presence of large anterior dorsal cingulate lesions (Cohen et al., [1999]; Swick and Turken, [2002]; Stemmer et al., 2004; Fellows and Farah [2005])."
For an evolving and now quite detailed alternative view of anterior cingulate, see Rushworth's review (2007). [20]
Reward based learning theory
A more comprehensive and recent theory describes the ACC as a more active component and poses that it detects and monitors errors, evaluates the degree of the error, and then suggests an appropriate form of action to be implemented by the motor system. Earlier evidence from electrical studies indicate the ACC has an evaluative component and this is indeed confirmed by fMRI studies. The dorsal and rostral areas of the ACC both seem to be affected by rewards and losses associated with errors. During one study, participants received monetary rewards and losses for correct and incorrect responses respectively.[14]
Largest activation in the dACC was shown during loss trials. This stimulus did not elicit any errors and thus error detection and monitoring theories cannot fully explain why this ACC activation would occur. The dorsal part of the ACC seems to play a key role in reward-based decision making and learning. The rostral part of the ACC, on the other hand, is believed to be more involved with affective responses to errors. In an interesting expansion of the previously described experiment, the effects of rewards and costs on ACC’s activation during error commission was examined.[16] Participants performed a version of the Eriksen flanker task using a set of letters assigned to each response button instead of arrows.
Targets were flanked by either a congruent or incongruent set of letters. Using an image of a thumb (up, down, or neutral) participants received feedback on how much money they gained or lost. The researchers found greater rostral ACC activation when participants lost money during the trials. The participants reported being frustrated when making mistakes. Because the ACC is intricately involved with error detection and affective responses, it may very well be that this area forms the bases of self-confidence. Taken together, these findings indicate that both the dorsal and rostral areas are involved in evaluating the extent of the error and optimizing subsequent responses. A study that confirming this notion explored the functions of both the dorsal and rostral areas of the ACC involved using a saccade task.[15]
Participants were shown a cue that indicated whether they had to make either a post saccade or an anti-saccade. An anti-saccade requires suppression of a distracting cue because the target appears in the opposite location causing the conflict. Results showed differing activation for the rostral and dorsal ACC areas. Early correct anti-saccade performance was associated with rostral activation. The dorsal area, on the other hand, was activated when errors were committed, but also for correct responses.
Whenever the dorsal area was active, fewer errors were committed providing more evidence that the ACC is involved with effortful performance. The second finding showed that during error trials, the ACC activated later than for correct responses clearly indicating a kind of evaluative function.
Incorporating the findings of the previously discussed studies, the rostral and dorsal areas of the ACC seem to be monitoring for errors and, when they occur, evaluate their severity. The ACC can then send a form of affective response based on the severity of the error and so provides feedback about what just happened and what to do next.
Pathology
Stimulation of the anterior cingulate (also known as Area 25) with low dosages of electric current in neurosurgical studies has been shown to improve depression in a portion of test subjects.
Studying the effects of damage to the ACC provides insights into the type of functions it serves in the intact brain. Behavior that is associated with lesions in the ACC includes: inability to detect errors, severe difficulty with resolving stimulus conflict in a Stroop task, emotional instability, inattention, and akinetic mutism.[1][2] There is evidence that damage to ACC is present in patients with schizophrenia, where studies have shown patients have difficulty in dealing with conflicting spatial locations in a Stroop-like task and having abnormal ERNs.[2][11] Participants with ADHD were found to have reduced activation in the dorsal area of the ACC when performing the Stroop task.[21] Together these findings corroborate results from imaging and electrical studies about the variety of functions attributed to the ACC.
There is evidence that this area may have a role in Obsessive Compulsive Disorder due to the fact that what appears to be an unnaturally low level of glutamate activity in this region has been observed in patients with the disorder,[22] in strange contrast to many other brain regions which are thought to have excessive glutamate activity in OCD.
Helen S. Mayberg and two collaborators described how they cured 4 of 6 depressed people—individuals virtually catatonic with depression despite years of talk therapy, drugs, even shock therapy—with pacemakerlike electrodes in area 25. A decade earlier Mayberg had identified area 25 as a key conduit of neural traffic between the "thinking" frontal cortex and the phylogenetically older central limbic region that gives rise to emotion. She subsequently found that area 25 appeared overactive in these depressed people — "like a gate left open," as she puts it — allowing negative emotions to overwhelm thinking and mood. Inserting the electrodes closed this gate and rapidly alleviated the depression of two-thirds of the trial's patients [23].
It has also been suggested to have possible links with Social Anxiety, along with the amygdala part of the brain, but is still in the early stages of research.
ACC and consciousness
The ACC area in the brain is associated with many functions that require conscious experience by the viewer. Higher ACC activation levels were found for more emotionally aware female participants when shown short ‘emotional’ video clips.[24] Better emotional awareness is associated with improved recognition of emotional cues or targets which is reflected by ACC activation.
The idea of awareness being associated with the ACC has some evidence with it, in that it seems to be the case that when subject’s responses are not congruent with actual responses, a larger ERN is produced.[12]
One study found an ERN even when subjects were not aware of their error.[12] Awareness may not be necessary to elicit an ERN, but it could influence the effect of the amplitude of the feedback ERN. Relating back to the reward based learning theory, awareness could modulate expectancy violations. Increased awareness could result in decreased violations of expectancies and decreased awareness could achieve the opposite effect. Further research is needed to completely understand the effects of awareness on ACC activation.
Relationship to Lead Poisoning
A 2008 study of brain MRI's taken on adults who had previously participated in the Cincinnati Lead Study demonstrated that people who had suffered higher levels of lead poisoning as children had decreased brain size as adults. This effect was most pronounced in the ACC (Cecil et al., 2008)[25] and is thought to relate to the cognitive and behavioral deficits of affected individuals.
الهامش
- ^ أ ب ت ث ج ح خ Bush G, Luu P, Posner MI (2000). "Cognitive and emotional influences in anterior cingulate cortex". Trends Cogn Sci. 4 (6): 215–222. doi:10.1016/S1364-6613(00)01483-2. PMID 10827444.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث ج Posner MI, DiGirolamo GJ (1998). "Executive attention: Conflict, target detection, and cognitive control". In Parasuraman R (ed.). The attentive brain. Cambridge, Mass: MIT Press. ISBN 0-262-16172-9.
- ^ Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001). "The anterior cingulate cortex. بروز وصلة بين العاطفة والإدراك". Ann N Y Acad Sci. 935: 107–17. PMID 11411161.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A (2001). "Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task". Psychophysiology. 38 (5): 752–60. doi:10.1017/S0048577201001111. PMID 11577898.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990). "The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm". Proc Natl Acad Sci USA. 87 (1): 256–9. doi:10.1073/pnas.87.1.256. PMC 53241. PMID 2296583.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Raichle ME, Fiez JA, Videen TO; et al. (1994). "Practice-related changes in human brain functional anatomy during nonmotor learning". Cereb. Cortex. 4 (1): 8–26. doi:10.1093/cercor/4.1.8. PMID 8180494.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gene blocker turns monkeys into workaholics
- ^ أ ب Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999). "Conflict monitoring versus selection-for-action in anterior cingulate cortex". Nature. 402 (6758): 179–81. doi:10.1038/46035. PMID 10647008.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Critchley HD (2005). "Neural mechanisms of autonomic, affective, and cognitive integration". J Comp Neurol. 493 (1): 154–66. doi:10.1002/cne.20749. PMID 16254997.
{{cite journal}}: Unknown parameter|month=ignored (help)
See Review by Critchely related to this - ^ Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993). "A neural system for error-detection and compensation". Psychological Science. 4 (6): 385–90. doi:10.1111/j.1467-9280.1993.tb00586.x.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث ج Holroyd CB, Nieuwenhuis S, Mars RB, Coles MGH (2004). "Anterior cingulate cortex, selection for action, and error processing". In Posner MI (ed.). Cognitive neuroscience of attention. New York: Guilford Press. pp. 219–31. ISBN 1-59385-048-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ أ ب ت ث ج ح خ Luu P, Pederson SM (2004). "The anterior cingulate cortex: Regulating actions in context". In Posner MI (ed.). Cognitive neuroscience of attention. New York: Guilford Press. ISBN 1-59385-048-4.
- ^ Gehring WJ, Knight RT (2000). "Prefrontal-cingulate interactions in action monitoring". Nat Neurosci. 3 (5): 516–20. doi:10.1038/74899. PMID 10769394.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ أ ب Bush G, Vogt BA, Holmes J; et al. (2002). "Dorsal anterior cingulate cortex: a role in reward-based decision making". Proc Natl Acad Sci USA. 99 (1): 523–8. doi:10.1073/pnas.012470999. PMC 117593. PMID 11756669.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS (2005). "Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission". Proc Natl Acad Sci USA. 102 (43): 15700–5. doi:10.1073/pnas.0503657102. PMC 1255733. PMID 16227444.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ أ ب Taylor SF, Martis B, Fitzgerald KD; et al. (2006). "Medial frontal cortex activity and loss-related responses to errors". J Neurosci. 26 (15): 4063–70. doi:10.1523/JNEUROSCI.4709-05.2006. PMID 16611823.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998). "Anterior cingulate cortex, error detection, and the online monitoring of performance". Science. 280 (5364): 747–9. doi:10.1126/science.280.5364.747. PMID 9563953.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Baird A, Dewar BK, Critchley H, Gilbert SJ, Dolan RJ, Cipolotti L (2006). "Cognitive functioning after medial frontal lobe damage including the anterior cingulate cortex: a preliminary investigation". Brain Cogn. 60 (2): 166–75. doi:10.1016/j.bandc.2005.11.003. PMID 16384629.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nachev P (2006). "Cognition and medial frontal cortex in health and disease". Curr Opin Neurol. 19 (6): 586–92. doi:10.1097/01.wco.0000247609.36482.ae. PMID 17102698.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rushworth MF, Behrens TE, Rudebeck PH, Walton ME (2007). "Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour". Trends Cogn Sci. 11 (4): 168–76. doi:10.1016/j.tics.2007.01.004. PMID 17337237.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bush G, Frazier JA, Rauch SL; et al. (1999). "Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop". Biol. Psychiatry. 45 (12): 1542–52. doi:10.1016/S0006-3223(99)00083-9. PMID 10376114.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pittenger C, Bloch M, Wegner R, Teitelbaum C, Krystal JH, Coric V (2006). "Glutamatergic Dysfunction in Obsessive-Compulsive Disorder and the Potential Clinical Utility of Glutamate-Modulating Agents". Primary Psychiatry. 13 (10): 65–77.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mayberg HS, Lozano AM, Voon V; et al. (2005). "Deep brain stimulation for treatment-resistant depression". Neuron. 45 (5): 651–60. doi:10.1016/j.neuron.2005.02.014. PMID 15748841.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE (1998). "Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex". J Cogn Neurosci. 10 (4): 525–35. doi:10.1162/089892998562924. PMID 9712681.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cecil KM, Brubaker CJ, Adler CM; et al. (2008). "Decreased brain volume in adults with childhood lead exposure". PLoS Med. 5 (5): e112. doi:10.1371/journal.pmed.0050112. PMID 18507499.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
- Goldapple K, Segal Z, Garson C; et al. (2004). "Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy". Arch Gen Psychiatry. 61 (1): 34–41. doi:10.1001/archpsyc.61.1.34. PMID 14706942.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
وصلات خارجية
- The Anterior Cingulate Cortex: The Evolution of an Interface Between Emotion and Cognition, paper by John Allman et al.
- allmanlab.caltech.edu - John Allman Lab
- TaipeiTimes.com - Know Thyself and Others
- قالب:BrainInfo
- Brain Implant Offers Hope for Severely Depressed
- Dobbs D (2006). "Turning Off Depression". Scientific American Mind. 17 (4): 26–31.
{{cite journal}}: Unknown parameter|month=ignored (help) - Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP (1998). "Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus". J Neurophysiol. 79 (4): 2231–4. PMID 9535984.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - NIF Search - Anterior Cingulate Cortex via the Neuroscience Information Framework