أحادي فلوريد الكلور
| |
| |
| الأسماء | |
|---|---|
| اسم أيوپاك
Chlorine monofluoride
| |
| أسماء أخرى
Chlorine fluoride
| |
| المُعرِّفات | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.300 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| الخصائص | |
| الصيغة الجزيئية | ClF |
| كتلة مولية | 54.45 g/mol |
| الكثافة | 1.62 g mL (liquid, −100 °C) |
| نقطة الانصهار | |
| نقطة الغليان | |
| البنية | |
| Dipole moment | 0.881 D (2.94 × 10−30 C m) |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−56.5 kJ mol−1 |
| Standard molar entropy S |
217.91 J K−1 mol−1 |
| سعة الحرارة النوعية، C | 33.01 J K−1 mol−1 |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula ClF. It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid. Many of its properties are intermediate between its parent halogens, Cl2 and F2.[1]
Geometry
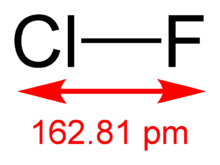
The molecular structure in the gas phase was determined by microwave spectroscopy; the bond length is re = 1.628341(4) Å.[2]
The bond length in the crystalline ClF is 1.628(1) Å; the lengthening relative to the free molecule is due to an interaction of the type F-Br···ClMe with a distance of 2.640(1) Å. In its molecular packing it shows very short intermolecular Cl···Cl contacts of 3.070(1) Å between neighboring molecules.[3]
Reactivity
Chlorine monofluoride is a versatile fluorinating agent, converting metals and non-metals to their fluorides and releasing Cl2 in the process. For example, it converts tungsten to tungsten hexafluoride and selenium to selenium tetrafluoride:
- W + 6 ClF → WF6 + 3 Cl2
- Se + 4 ClF → SeF4 + 2 Cl2
FCl can also chlorofluorinate compounds, either by addition across a multiple bond or via oxidation. For example, it adds fluorine and chlorine to the carbon of carbon monoxide, yielding carbonyl chloride fluoride:
انظر أيضاً
References
- ^ Otto Ruff, E. Ascher (1928). "Über ein neues Chlorfluorid-CIF3". Zeitschrift für anorganische und allgemeine Chemie. 176 (1): 258–270. doi:10.1002/zaac.19281760121.
- ^ Willis, Robert E.; Clark, William W. (May 1980). "Millimeter wave measurements of the rotational spectra of ClF, BrF, BrCl, ICl, and IBr". The Journal of Chemical Physics (in الإنجليزية). 72 (9): 4946–4950. Bibcode:1980JChPh..72.4946W. doi:10.1063/1.439780. ISSN 0021-9606.
- ^ Boese, Roland; Boese, A. Daniel; Bláser, Dieter; Antipin, Michail Yu.; Ellern, Arkadi; Seppelt, Konrad (1997-08-04). "The Surprising Crystal Packing of Chlorinefluoride". Angewandte Chemie International Edition in English. 36 (1314): 1489–1492. doi:10.1002/anie.199714891. ISSN 0570-0833.
وصلات خارجية
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles containing unverified chemical infoboxes
- Short description is different from Wikidata
- Fluorides
- Fluorinating agents
- Inorganic chlorine compounds
- Interhalogen compounds
- Diatomic molecules
- عوامل مؤكسدة