حالة مستقرة
| جزء من سلسلة مقالات عن |
| ميكانيكا الكم |
|---|
الحالة المستقرة stationary state هي حالة كمية يكون فيها كل القابلين للرصد مستقلين عن الزمن. وهي متجه ذاتي للهاملتونية.[1] وهذه تناظر حالة بطاقة محددة منفردة (بدلاً من تراكب كمي لطاقات مختلفة). كما تـُدعى متجه ذاتي للطاقة، حالة ذاتية للطاقة، دالة ذاتية للطاقة، أو كت ذاتي للطاقة. وهي شبيهة جداً لمفهوم مدار ذري و مدار جزيئي في الكيمياء، مع بعض الاختلافات الطفيفة المشروحة أدناه.
الحالة الأرضية في نظام ميكانيكا الكم تعني أقل حالات الطاقة. و تكون الحالة المثارة هي أي حالة لها طاقة أعلى من الحالة الأرضية. الحالة الأرضية في نظرية مجال الكم غالبا ما يطلق عليها حالة فراغ أو الفراغ.
في حالة تواجد أكثر من حالة أرضية، يقال أنهم في حالة انحلال. و يوجد العديد من الأنظمة التي بها هذه الحالة، مثل ذرة الهيدروجين. اتضح أن الانحلال يحدث عند الحادث غير العادي للعامل الوحيد استبدل بهاملتونية النظام.
وطبقا لقانون الديناميكا الحرارية الثالث، فالنظام الموجود في درجة حرارة الصفر المطلق يكون في حالته الأرضية، و على ذلك، فإن الإنتروبي الخاص به يتم تحديده بتفسخ الحالة الأرضية. وعديد من الأنظمة مثل الشبكات البلورية، لها حالة أرضية واحدة و على هذا يكون لها صفر إنتروبي عند الصفر المطلق لأن ln(1) = 0.
مقدمة

A stationary state is called stationary because the system remains in the same state as time elapses, in every observable way. For a single-particle Hamiltonian, this means that the particle has a constant probability distribution for its position, its velocity, its spin, etc.[2] (This is true assuming the particle's environment is also static, i.e. the Hamiltonian is unchanging in time.) The wavefunction itself is not stationary: It continually changes its overall complex phase factor, so as to form a standing wave. The oscillation frequency of the standing wave, times Planck's constant, is the energy of the state according to the Planck–Einstein relation.
Stationary states are quantum states that are solutions to the time-independent Schrödinger equation:
حيث
- is a quantum state, which is a stationary state if it satisfies this equation;
- is the Hamiltonian operator;
- is a real number, and corresponds to the energy eigenvalue of the state .
This is an eigenvalue equation: is a linear operator on a vector space, is an eigenvector of , and is its eigenvalue.
If a stationary state is plugged into the time-dependent Schrödinger Equation, the result is:[3]
Assuming that is time-independent (unchanging in time), this equation holds for any time t. Therefore, this is a differential equation describing how varies in time. Its solution is:
Therefore, a stationary state is a standing wave that oscillates with an overall complex phase factor, and its oscillation angular frequency is equal to its energy divided by .
خصائص الحالة الأرضية
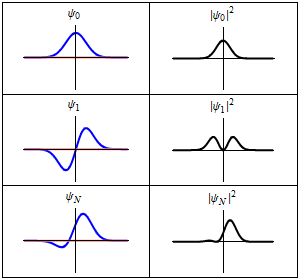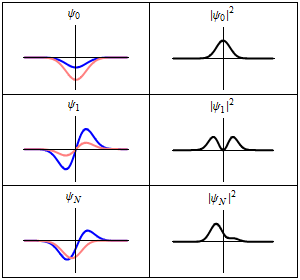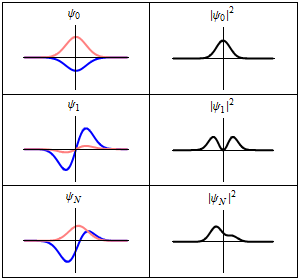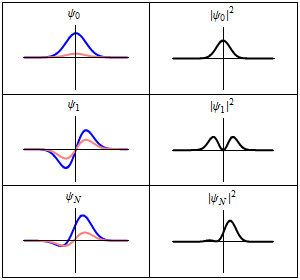
As shown above, a stationary state is not mathematically constant:
However, all observable properties of the state are in fact constant in time. For example, if represents a simple one-dimensional single-particle wavefunction , the probability that the particle is at location x is:
which is independent of the time t.
The Heisenberg picture is an alternative mathematical formulation of quantum mechanics where stationary states are truly mathematically constant in time.
As mentioned above, these equations assume that the Hamiltonian is time-independent. This means simply that stationary states are only stationary when the rest of the system is fixed and stationary as well. For example, a 1s electron in a hydrogen atom is in a stationary state, but if the hydrogen atom reacts with another atom, then the electron will of course be disturbed.
الاضمحلال الفوري
Spontaneous decay complicates the question of stationary states. For example, according to simple (nonrelativistic) quantum mechanics, the hydrogen atom has many stationary states: 1s, 2s, 2p, and so on, are all stationary states. But in reality, only the ground state 1s is truly "stationary": An electron in a higher energy level will spontaneously emit one or more photons to decay into the ground state.[4] This seems to contradict the idea that stationary states should have unchanging properties.
The explanation is that the Hamiltonian used in nonrelativistic quantum mechanics is only an approximation to the Hamiltonian from quantum field theory. The higher-energy electron states (2s, 2p, 3s, etc.) are stationary states according to the approximate Hamiltonian, but not stationary according to the true Hamiltonian, because of vacuum fluctuations. On the other hand, the 1s state is truly a stationary state, according to both the approximate and the true Hamiltonian.
المقارنة مع "المدار" في الكيمياء
انظر أيضا
الهامش
- ^ Quantum Mechanics Demystified, D. McMahon, Mc Graw Hill (USA), 2006, ISBN 0-07-145546-9
- ^ Cohen-Tannoudji, Claude, Bernard Diu, and Franck Laloë. Quantum Mechanics: Volume One. Hermann, 1977. p. 32.
- ^ Quanta: A handbook of concepts, P.W. Atkins, Oxford University Press, 1974, ISBN 0-19-855493-1
- ^ Quantum Physics of Atoms, Molecules, Solids, Nuclei, and Particles (2nd Edition), R. Eisberg, R. Resnick, John Wiley & Sons, 1985, ISBN 978-0-471-87373-0
للاستزادة
- Stationary states, Alan Holden, Oxford University Press, 1971, ISBN 0-19-851121-3











