گلايسين
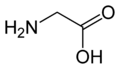
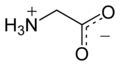
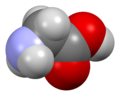
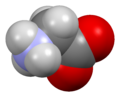
الگلايسين Glycine (اختصاره Gly أو G)[6]، هو مركب عضوي صيغته الكيميائية NH2CH2COOH. بامتلاكه مستبدل هيدروجين كما في سلسلته الجانبية، يعتبر الگلايسين الأصغر بين الأحماض الأمينية العشرين الشائعة في الپروتينات، وفي الواقع هو الأصغر المرجح. كودونات شفرته الوراثية هي GGU, GGC, GGA, GGG.
| |||
| |||
| |||
| الأسماء | |||
|---|---|---|---|
| اسم أيوپاك
Glycine
| |||
| اسم أيوپاك النظامي
Aminoacetic acid[2] | |||
| أسماء أخرى
2-Aminoethanoic acid
Glycocol Glycic acid Dicarbamic acid | |||
| Identifiers | |||
| رقم CAS | |||
3D model (JSmol)
|
|||
| اختصارات | Gly, G | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.248 | ||
| رقم EC |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| InChI | InChI={{{value}}} | ||
| SMILES | |||
| الخصائص | |||
| الصيغة الجزيئية | C2H5NO2 | ||
| كتلة مولية | 75.06 g mol-1 | ||
| المظهر | White solid | ||
| الكثافة | 1.1607 g/cm3[3] | ||
| نقطة الانصهار | |||
| قابلية الذوبان في الماء | 24.99 g/100 mL (25 °C)[4] | ||
| قابلية الذوبان | قابل للذوبان في الپيريدين قابل للذبان بشكل معتدل في الإيثانول غير قابل للذوبان في الإيثر | ||
| الحموضة (pKa) | 2.34 (carboxyl), 9.6 (amino)[5] | ||
| القابلية المغناطيسية | -40.3·10−6 cm3/mol | ||
| المخاطر | |||
| الجرعة أو التركيز القاتل (LD, LC): | |||
LD50 (الجرعة الوسطى)
|
2600 mg/kg (mouse, oral) | ||
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |||
| مراجع الجدول | |||
الگلايسين هو مادة صلبة بلورية عديمة اللون، حلوة المذاق. يعتبر فريداً بين الأحماض الأمينية المركبة للپروتينات حيث يعتبر كيرالياً. يمكنه أن يتلائم مع البيئات المحبة للماء أو الكارهة للماء، بسبب سلسلته الجانبية الدنيا التي تحتوي على ذرة هيدروجين واحدة. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.
It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التاريخ وأصل الاسم
Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid.[7] He originally called it "sugar of gelatin",[8][9] but French chemist Jean-Baptiste Boussingault showed in 1838 that it contained nitrogen.[10] In 1847 American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig, proposed the name "glycocoll";[11][12] however, the Swedish chemist Berzelius suggested the simpler current name a year later.[13][14] The name comes from the Greek word γλυκύς "sweet tasting"[15] (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose). In 1858, the French chemist Auguste Cahours determined that glycine was an amine of acetic acid.[16]
الانتاج
Although glycine can be isolated from hydrolyzed protein, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis.[17] The two main processes are amination of chloroacetic acid with ammonia, giving glycine and ammonium chloride,[18] and the Strecker amino acid synthesis,[19] which is the main synthetic method in the United States and Japan.[20] About 15 thousand tonnes are produced annually in this way.[21]
Glycine is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.[22]
التفاعلات الكيميائية
Its acid–base properties are most important. In aqueous solution, glycine is amphoteric: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about 9.6, it converts to glycinate.
Glycine functions as a bidentate ligand for many metal ions, forming amino acid complexes. A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which exists both in cis and trans isomers.
With acid chlorides, glycine converts to the amidocarboxylic acid, such as hippuric acid[23] and acetylglycine.[24] With nitrous acid, one obtains glycolic acid (van Slyke determination). With methyl iodide, the amine becomes quaternized to give trimethylglycine, a natural product:
- H 3N+ CH 2COO− + 3 CH3I → (CH 3) 3N+ CH 2COO− + 3 HI
Glycine condenses with itself to give peptides, beginning with the formation of glycylglycine:
- 2 H 3N+ CH 2COO− → H 3N+ CH 2CONHCH 2COO− + H2O
Pyrolysis of glycine or glycylglycine gives 2,5-diketopiperazine, the cyclic diamide.
It forms esters with alcohols. They are often isolated as their hydrochloride, e.g., glycine methyl ester hydrochloride. Otherwise the free ester tends to convert to diketopiperazine.
As a bifunctional molecule, glycine reacts with many reagents. These can be classified into N-centered and carboxylate-center reactions.
الأيض
التخليق الحيوي
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate, but one publication made by supplements sellers seems to show that the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis.[25] In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[26]
- serine + tetrahydrofolate → glycine + N5,N10-methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:[26]
- CO2 + NH+4 + N5,N10-methylene tetrahydrofolate + NADH + H+ ⇌ Glycine + tetrahydrofolate + NAD+
In addition to being synthesized from serine, glycine can also be derived from threonine, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.[27]
التحلل
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above. In this context, the enzyme system involved is usually called the glycine cleavage system:[26]
- Glycine + tetrahydrofolate + NAD+ ⇌ CO2 + NH+4 + N5,N10-methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.[26]
In the third pathway of its degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.[26]
The half-life of glycine and its elimination from the body varies significantly based on dose.[28] In one study, the half-life varied between 0.5 and 4.0 hours.[28]
Glycine is extremely sensitive to antibiotics which target folate, and blood glycine levels drop severely within a minute of antibiotic injections. Some antibiotics can deplete more than 90% of glycine within a few minutes of being administered.[29]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الوظيفة الفسيولوجية
The principal function of glycine is it acts as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction with hydroxyproline.[26][30] In the genetic code, glycine is coded by all codons starting with GG, namely GGU, GGC, GGA and GGG.
كوسيط مخلق حيوياً
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.[26]
كناقل عصبي
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory.[31] The ج.م.50 of glycine is 7930 mg/kg in rats (oral),[32] and it usually causes death by hyperexcitability.
الاستخدامات
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which may or may not meet USP grade standards, is sold at a lower price for use in industrial applications, e.g., as an agent in metal complexing and finishing.[33]
طعام الحيوان والإنسان
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.[21]
The U.S. "Food and Drug Administration no longer regards glycine and its salts as generally recognized as safe for use in human food".[35]
أعلاف كيميائية
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicides glyphosate,[36] iprodione, glyphosine, imiprothrin, and eglinazine.[21] It is used as an intermediate of the medicine such as thiamphenicol.[بحاجة لمصدر]
الأبحاث المعملية
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis. Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required. This process is known as stripping.
الوجود في الفضاء
The presence of glycine outside the earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft Stardust from comet Wild 2 and subsequently returned to earth. Glycine had previously been identified in the Murchison meteorite in 1970.[37] The discovery of glycine in outer space bolstered the hypothesis of so called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe.[38] In 2016, detection of glycine within Comet 67P/Churyumov–Gerasimenko by the Rosetta spacecraft was announced.[39]
The detection of glycine outside the Solar System in the interstellar medium has been debated.[40] In 2008, the Max Planck Institute for Radio Astronomy discovered the spectral lines of a glycine precursor (aminoacetonitrile) in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius.[41]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التطور
Glycine is proposed to be defined by early genetic codes.[42][43][44][45] For example, low complexity regions (in proteins), that may resemble the proto-peptides of the early genetic code are highly enriched in glycine.[45]
التواجد في الطعام
| Food | g/100g |
|---|---|
| Snacks, pork skins | 11.04 |
| Sesame seeds flour (low fat) | 3.43 |
| Beverages, protein powder (soy-based) | 2.37 |
| Seeds, safflower seed meal, partially defatted | 2.22 |
| Meat, bison, beef and others (various parts) | 1.5-2.0 |
| Gelatin desserts | 1.96 |
| Seeds, pumpkin and squash seed kernels | 1.82 |
| Turkey, all classes, back, meat and skin | 1.79 |
| Chicken, broilers or fryers, meat and skin | 1.74 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 1.71 |
| Bacon and beef sticks | 1.64 |
| Peanuts | 1.63 |
| Crustaceans, spiny lobster | 1.59 |
| Spices, mustard seed, ground | 1.59 |
| Salami | 1.55 |
| Nuts, butternuts, dried | 1.51 |
| Fish, salmon, pink, canned, drained solids | 1.42 |
| Almonds | 1.42 |
| Fish, mackerel | 0.93 |
| Cereals ready-to-eat, granola, homemade | 0.81 |
| Leeks, (bulb and lower-leaf portion), freeze-dried | 0.7 |
| Cheese, parmesan (and others), grated | 0.56 |
| Soybeans, green, cooked, boiled, drained, without salt | 0.51 |
| Bread, protein (includes gluten) | 0.47 |
| Egg, whole, cooked, fried | 0.47 |
| Beans, white, mature seeds, cooked, boiled, with salt | 0.38 |
| Lentils, mature seeds, cooked, boiled, with salt | 0.37 |
انظر أيضاً
المصادر
- ^ قالب:Merck11th
- ^ pubchem.ncbi.nlm.nih.gov/compound/750#section=IUPAC-Name&fullscreen=true
- ^ Handbook of Chemistry and Physics, CRC Press, 59th edition, 1978
- ^ "Solubilities and densities". Prowl.rockefeller.edu. Archived from the original on 2017-09-12. Retrieved 2013-11-13.
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ قالب:IUPAC-IUB amino acids 1983.
- ^ Plimmer, R.H.A. (1912) [1908]. Plimmer, R.H.A.; Hopkins, F.G. (eds.). The chemical composition of the proteins. Monographs on biochemistry. Vol. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 82. Retrieved January 18, 2010.
- ^ Braconnot, Henri (1820). "Sur la conversion des matières animales en nouvelles substances par le moyen de l'acide sulfurique" [On the conversion of animal materials into new substances by means of sulfuric acid]. Annales de Chimie et de Physique. 2nd series (in الفرنسية). 13: 113–125. ; see p. 114.
- ^ MacKenzie, Colin (1822). One Thousand Experiments in Chemistry: With Illustrations of Natural Phenomena; and Practical Observations on the Manufacturing and Chemical Processes at Present Pursued in the Successful Cultivation of the Useful Arts … (in الإنجليزية). Sir R. Phillips and Company. p. 557.
- ^ Boussingault (1838). "Sur la composition du sucre de gélatine et de l'acide nitro-saccharique de Braconnot" [On the composition of sugar of gelatine and of nitro-glucaric acid of Braconnot]. Comptes Rendus (in الفرنسية). 7: 493–495.
- ^ Horsford, E.N. (1847). "Glycocoll (gelatine sugar) and some of its products of decomposition". The American Journal of Science and Arts. 2nd series. 3: 369–381.
- ^ Ihde, Aaron J. (1970). The Development of Modern Chemistry (in الإنجليزية). Courier Corporation. ISBN 9780486642352.
- ^ Berzelius, Jacob (1848). Jahres-Bericht über die Fortschritte der Chemie und Mineralogie (Annual Report on the Progress of Chemistry and Mineralogy). Vol. 47. Tübigen, (Germany): Laupp. p. 654. From p. 654: "Er hat dem Leimzucker als Basis den Namen Glycocoll gegeben. … Glycin genannt werden, und diesen Namen werde ich anwenden." (He [i.e., the American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig] gave the name "glycocoll" to Leimzucker [sugar of gelatine], a base. This name is not euphonious and has besides the flaw that it clashes with the names of the rest of the bases. It is compounded from γλυχυς (sweet) and χολλα (animal glue). Since this organic base is the only [one] which tastes sweet, then it can much more briefly be named "glycine", and I will use this name.)
- ^ Nye, Mary Jo (1999). Before Big Science: The Pursuit of Modern Chemistry and Physics, 1800-1940 (in الإنجليزية). Harvard University Press. ISBN 9780674063822.
- ^ "glycine". Oxford Dictionaries. Archived from the original on November 13, 2014. Retrieved 2015-12-06.
- ^ Cahours, A. (1858). "Recherches sur les acides amidés" [Investigations into aminated acids]. Comptes Rendus (in الفرنسية). 46: 1044–1047.
- ^ Okafor, Nduka (2016-03-09). Modern Industrial Microbiology and Biotechnology (in الإنجليزية). CRC Press. ISBN 9781439843239.
- ^ قالب:OrgSynth
- ^ Wiley (2007-12-14). Kirk-Othmer Food and Feed Technology, 2 Volume Set (in الإنجليزية). John Wiley & Sons. ISBN 9780470174487.
- ^ "Glycine Conference (prelim)". USITC. Archived from the original on 2012-02-22. Retrieved 2014-06-13.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ أ ب ت Drauz, Karlheinz; Grayson, Ian; Kleemann, Axel; Krimmer, Hans-Peter; Leuchtenberger, Wolfgang & Weckbecker, Christoph (2007). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ Hart, J. Roger (2005). "Ethylenediaminetetraacetic Acid and Related Chelating Agents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_095.
{{cite encyclopedia}}: Cite has empty unknown parameter:|authors=(help) - ^ Ingersoll, A. W.; Babcock, S. H. (1932). "Hippuric Acid". Org. Synth. 12: 40. doi:10.15227/orgsyn.012.0040.
- ^ Herbst, R. M.; Shemin, D. (1939). "Acetylglycine". Org. Synth. 19: 4. doi:10.15227/orgsyn.019.0004.
- ^ Meléndez-Hevia, E; De Paz-Lugo, P; Cornish-Bowden, A; Cárdenas, M. L. (December 2009). "A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis". Journal of Biosciences. 34 (6): 853–72. doi:10.1007/s12038-009-0100-9. PMID 20093739. S2CID 2786988.
- ^ أ ب ت ث ج ح خ Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, pp. 127, 675–77, 844, 854, ISBN 0-7167-4339-6
- ^ Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. (2013). "Glycine metabolism in animals and humans: Implications for nutrition and health". Amino Acids. 45 (3): 463–77. doi:10.1007/s00726-013-1493-1. PMID 23615880. S2CID 7577607.
- ^ أ ب Hahn RG (1993). "Dose-dependent half-life of glycine". Urological Research. 21 (4): 289–291. doi:10.1007/BF00307714. PMID 8212419. S2CID 25138444.
- ^ ACS Chem Biol. 2010 Aug 20; 5(8): 787–795. doi: 10.1021/cb100096f
- ^ Szpak, Paul (2011). "Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope analysis". Journal of Archaeological Science. 38 (12): 3358–3372. doi:10.1016/j.jas.2011.07.022.
- ^ "Recent development in NMDA receptors". Chinese Medical Journal. 2000.
- ^ "Safety (MSDS) data for glycine". The Physical and Theoretical Chemistry Laboratory Oxford University. 2005. Archived from the original on 2007-10-20. Retrieved 2006-11-01.
- ^ "Glycine From Japan and Korea" (PDF). U.S. International Trade Commission. January 2008. Archived (PDF) from the original on 2010-06-06. Retrieved 2014-06-13.
- ^ Casari, B. M.; Mahmoudkhani, A. H.; Langer, V. (2004). "A Redetermination of cis-Aquabis(glycinato-κ2N,O)copper(II)". Acta Crystallogr. E. 60 (12): m1949–m1951. doi:10.1107/S1600536804030041.
- ^ "eCFR :: 21 CFR 170.50 -- Glycine (aminoacetic acid) in food for human consumption". ecfr.gov. Retrieved 2022-10-24.
- ^ Stahl, Shannon S.; Alsters, Paul L. (2016-07-13). Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives (in الإنجليزية). John Wiley & Sons. ISBN 9783527690152.
- ^ Kvenvolden, Keith A.; Lawless, James; Pering, Katherine; Peterson, Etta; Flores, Jose; Ponnamperuma, Cyril; Kaplan, Isaac R.; Moore, Carleton (1970). "Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite". Nature. 228 (5275): 923–926. Bibcode:1970Natur.228..923K. doi:10.1038/228923a0. PMID 5482102. S2CID 4147981.
- ^ "Building block of life found on comet - Thomson Reuters 2009". Reuters. 18 August 2009. Retrieved 2009-08-18.
- ^ European Space Agency (27 May 2016). "Rosetta's comet contains ingredients for life". Retrieved 2016-06-05.
- ^ Snyder LE, Lovas FJ, Hollis JM, et al. (2005). "A rigorous attempt to verify interstellar glycine". Astrophys J. 619 (2): 914–930. arXiv:astro-ph/0410335. Bibcode:2005ApJ...619..914S. doi:10.1086/426677. S2CID 16286204.
- ^ Staff. "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius 27 March 2008 - Science Daily". Retrieved 2008-09-16.
- ^ Trifonov, E.N (December 2000). "Consensus temporal order of amino acids and evolution of the triplet code". Gene (in الإنجليزية). 261 (1): 139–151. doi:10.1016/S0378-1119(00)00476-5. PMID 11164045.
- ^ Higgs, Paul G.; Pudritz, Ralph E. (June 2009). "A Thermodynamic Basis for Prebiotic Amino Acid Synthesis and the Nature of the First Genetic Code". Astrobiology (in الإنجليزية). 9 (5): 483–490. arXiv:0904.0402. Bibcode:2009AsBio...9..483H. doi:10.1089/ast.2008.0280. ISSN 1531-1074. PMID 19566427. S2CID 9039622.
- ^ Chaliotis, Anargyros; Vlastaridis, Panayotis; Mossialos, Dimitris; Ibba, Michael; Becker, Hubert D.; Stathopoulos, Constantinos; Amoutzias, Grigorios D. (2017-02-17). "The complex evolutionary history of aminoacyl-tRNA synthetases". Nucleic Acids Research (in الإنجليزية). 45 (3): 1059–1068. doi:10.1093/nar/gkw1182. ISSN 0305-1048. PMC 5388404. PMID 28180287.
- ^ أ ب Ntountoumi, Chrysa; Vlastaridis, Panayotis; Mossialos, Dimitris; Stathopoulos, Constantinos; Iliopoulos, Ioannis; Promponas, Vasilios; Oliver, Stephen G; Amoutzias, Grigoris D (2019-11-04). "Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved". Nucleic Acids Research (in الإنجليزية). 47 (19): 9998–10009. doi:10.1093/nar/gkz730. ISSN 0305-1048. PMC 6821194. PMID 31504783.
- ^ "National Nutrient Database for Standard Reference". U.S. Department of Agriculture. Archived from the original on 2015-03-03. Retrieved 2009-09-07.
{{cite journal}}: Cite journal requires|journal=(help)
قراءات إضافية
On attempts to detect glycine in interstellar medium
- Kuan YJ, Charnley SB, Huang HC; et al. (2003). "Interstellar glycine". Astrophys J. 593 (2): 848–867. Bibcode:2003ApJ...593..848K. doi:10.1086/375637.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Rachel Nowak. "Amino acid found in deep space - 18 July 2002 - New Scientist". Retrieved 2007-07-01.
وصلات خارجية
- Glycine MS Spectrum
- Glycine at PDRHealth.com
- Glycine cleavage system
- Glycine Therapy - A New Direction for Schizophrenia Treatment?
- "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius". ScienceDaily. 27 March 2008.
- Guochuan E. Tsai (1 December 2008). "A New Class of Antipsychotic Drugs: Enhancing Neurotransmission Mediated by NMDA Receptors". Psychiatric Times. 25 (14).
- ChemSub Online (Glycine).
- NASA scientists have discovered glycine, a fundamental building block of life, in samples of comet Wild 2 returned by NASA's Stardust spacecraft.