المكثف الكهربائي مزدوج الطبقة

المكثفات الكهربائية مزدوجة الطبقة , تعرف أيضا supercapacitors, كهربائية مزدوجة الطبقة المكثفات (EDLCs), أو ultracapacitors, هى كهرو كيميائية مكثفات وهى تمتلك كثافة طاقة عالية بدرجة غير عادية عندما تقارن بالمكثفات العادية, عادة بناء على أمر عدة آلاف من المرات أكبر من المكثفات الكهربائية عالية القدرة مثلا, نموذجية مكثف الحجم الكهربائي D خلية مكثف سيكون لها سعة في مجموعة من عشرات الميللى فاراد. نفس الجهد الكهربائي لمكثف مزدوج الطبقة موسعة سيكون له عدة فارادات ، وهو ما يمثل تحسنا عن اثنين أو ثلاثة [من حيث الحجم]] في السعة, ولكن في الغالب عند فرق جهد أقل عمليا .[1] أعلى كثافة للطاقة في التشغيل هى 30 Wh/كج.[2] المكثف الكهربائي مزدوج الطبقة يهدف الى ملىء الفجوة بين المكثفات و البطاريات [3].
المكثفات الكهربائية مزدوجة الطبقة لها إستحدامات تجارية عديدة, تتضح أكثر في "تمهيد الطاقة" و أدوات التحميل-اللحظى. بعض أقرب الاستخدامات المكثفات لبدء المحركات الكبيرة في للدبابات و الغواصات ,و حينما تهاوت الأسعار, بدأت تظهر تلك المكثفات في شاحنات الديزيل و قاطرات السكك الحديدية.[4] و حديثا اصبحت تلك المكثفات, موضوعا ذى أهمية في عالم الطاقة الخضراء ، حيث قدرتها على امتصاص الطاقة سرعان ما يجعلها مناسبة لتطبيقات [الكوابح المتجددة] , حيث أن البطاريات تجد صعوبة في هذا تطبيق بسبب بطء معدلات الشحن. تكنولوجيا جديدة في مجال التطوير يمكن أن تجعل EDLC مع كثافة عالية من الطاقة ما يكفي ليكون جذابا لاستبدال البطاريات في جميع السيارات الكهربائية و الإضافات الهجين مثل EDLCs هى سريعة الشحن وتظهر ثباتا كهربائيا. و يمكن إستخدامها أيضا في كروت الحاسب ,معدات الفلاش تصوير ضوئى في الكاميرات الرقمية, آلات العروض المحمولة, وفى [[قراءة العداد الآلي ]] [5].
ماهية الموضوع
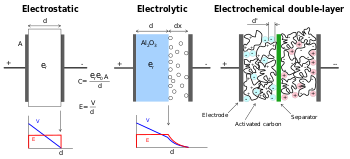
فى المكثف المعتاد, ويتم تخزين الطاقة من خلال إزالة حاملات الشحنة, أى الإليكترونات من, من القطب المعدنى ووضعه على القطب الآخر. وهذا الفصل بين الشحنة يفسح المجال لفرق في الجهد يظهر بين القطبين , والتي يمكن تسخيرها في الدائرة الخارجية. و كان مجموع الطاقة المخزنة على هذا النحو يتناسب مع كلا من عدد الشحنات المخزنة و فرق الجهد بين القطبين. و الأول يعبر أساسا عن معادلة بين الحجم , خواص مادة القطبين , و الأخير يكون محدودا بسبب العزل التحطم بين القطبين. المواد المختلفة التي تقع بين لوحات للفصل بينهما ينتج عنها فرق غى الجهد مختلف ينبغى تخزينه. البحث عن مواد مثلى يؤدي إلى ارتفاع كثافة الطاقة لأي حجم مكثف. In contrast with traditional capacitors, electric double-layer capacitors do not have a conventional dielectric. Rather than two separate plates separated by an intervening substance, these capacitors use "plates" that are in fact two layers of the same substrate, and their electrical properties, the so-called "electrical double layer", result in the effective separation of charge despite the vanishingly thin (on the order of nanometers) physical separation of the layers. The lack of need for a bulky layer of dielectric permits the packing of "plates" with much larger surface area into a given size, resulting in their extraordinarily high capacitances in practical sized packages.
In an electrical double layer, each layer by itself is quite conductive, but the physics at the interface where the layers are effectively in contact means that no significant current can flow between the layers. However, the double layer can withstand only a low voltage, which means that electric double-layer capacitors rated for higher voltages must be made of matched series-connected individual electric double-layer capacitors, much like series-connected cells in higher-voltage batteries.
In general, electric double-layer capacitors improve storage density through the use of a nanoporous material, typically activated charcoal, in place of the conventional insulating barrier. Activated charcoal is a powder made up of extremely small and very "rough" particles, which in bulk form a low-density volume of particles with holes between them that resembles a sponge. The overall surface area of even a thin layer of such a material is many times greater than a traditional material like aluminum, allowing many more charge carriers (ions or radicals from the electrolyte) to be stored in any given volume. The downside is that the charcoal is taking the place of the improved insulators used in conventional devices, so in general electric double-layer capacitors use low potentials on the order of 2 to 3 V.
Activated charcoal is not the "perfect" material for this application. The charge carriers are actually (in effect) quite large - especially when surrounded by solvent molecules - and are often larger than the holes left in the charcoal, which are too small to accept them, limiting the storage. Recent research in electric double-layer capacitors has generally focused on improved materials that offer even higher usable surface areas. Experimental devices developed at MIT replace the charcoal with carbon nanotubes, which have similar charge storage capability as charcoal (which is almost pure carbon) but are mechanically arranged in a much more regular pattern that exposes a much greater suitable surface area.[6] Other teams are experimenting with custom materials made of activated polypyrrole, and even nanotube-impregnated papers.
In terms of energy density, existing commercial electric double-layer capacitors range around 0.5 to 30 W·h/kg, with the standardized cells available from Maxwell Technologies rated at 6 W·h/kg and ACT in production of 30 Wh/kg. [7][8] Note however that ACT's capacitor is actually a Lithium_ion_capacitor, known also as a "hybrid capacitor". Experimental electric double-layer capacitors from the MIT LEES project have demonstrated densities of 30 W·h/kg and appear to be scalable to 60 W·h/kg in the short term,[9] while EEStor claims their examples will offer capacities about 400 W·h/kg. For comparison, a conventional lead-acid battery is typically 30 to 40 W·h/kg and modern lithium-ion batteries are about 160 W·h/kg. In automobile applications gasoline has a net calorific value (NCV) of around 12,000 W·h/kg, which operates at 20% tank-to-wheel efficiency giving an effective energy density of 2,400 W·h/kg.
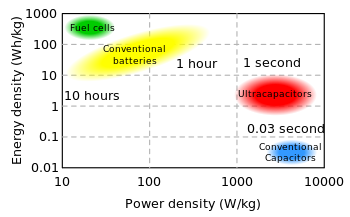
Additionally, electric double-layer capacitors offer much higher power density than batteries. Power density combines the energy density with the speed that the energy can be drawn out of the device. Batteries, which are based on the movement of charge carriers in a liquid electrolyte, have relatively slow charge and discharge times. Capacitors, on the other hand, can be charged or discharged at a rate that is typically limited by current heating of the electrodes. So while existing electric double-layer capacitors have energy densities that are perhaps 1/10th that of a conventional battery, their power density is generally ten to one-hundred times as great (see diagram, right).
التاريخ
تأثير المكثف الكهربائي مزدوج الطبقة لوحظ للأول مرة في 1957 بواسطة مهندسي چنرال إليكتريك أجروا تجاربا مع الأجهزة التي تستخدم الكربون المسامي كقطب كهربائي .[10] و قد أعتقد أن الطاقة يتم تخزينها, في المسام الكربونية و قد أظهرت بصفة إستثنائية تكثيفا عاليا", بالرغم أن الآلية لم تتضح في ذلك الحين.
General Electric did not immediately follow up on this work, and the modern version of the devices were eventually developed by researchers at Standard Oil of Ohio in 1966, after they accidentally re-discovered the effect while working on experimental fuel cell designs.[11] Their cell design used two layers of activated charcoal separated by a thin porous insulator, and this basic mechanical design remains the basis of most electric double-layer capacitors to this day.
Standard Oil also failed to commercialize their invention, licensing the technology to NEC, who finally marketed the results as “supercapacitors” in 1978, to provide backup power for maintaining computer memory.[11] The market expanded slowly for a time, but starting around the mid-1990s various advances in materials science and simple development of the existing systems led to rapidly improving performance and an equally rapid reduction in cost.
In 2005, the ultracapacitor market was between US $272 million and $400 million, depending on the source. It is rapidly growing, especially in the automotive sector.[11]
Recently [12], all solid state micrometer-scale electric double-layer capacitors based on advanced superionic conductors had been recognized as critical electron components of future sub-voltage and deep-sub-voltage nanoelectronics and related technologies (22 nm technological node of CMOS and beyond).
Technology advantages
Due to the capacitor's high number of charge-discharge cycles (millions or more compared to 200-1000 for most commercially available rechargeable batteries) there are no disposable parts during the whole operating life of the device, which makes the device environmentally friendly. Batteries wear out on the order of a few years, and their highly reactive chemical electrolytes present a serious disposal and safety hazard. This can be improved by only charging under favorable conditions, at an ideal rate, and, for some chemistries, as infrequently as possible. Electric double-layer capacitors can help in this regard, acting as a charge conditioner, storing energy from other sources for load balancing purposes and then using any excess energy to charge the batteries only at opportune times.
Other advantages of electric double-layer capacitors compared with rechargeable batteries are extremely low internal resistance or ESR, high efficiency (up to 97-98%), high output power, extremely low heating levels, and improved safety. According to ITS (Institute of Transportation Studies, Davis, CA) test results, the specific power of electric double-layer capacitors can exceed 6 kW/kg at 95% efficiency [13]
The idea of replacing batteries with capacitors in conjunction with novel alternative energy sources became a conceptual umbrella of the Green Electricity (GEL) Initiative [2], [3], introduced by Dr. Alexander Bell. One particular successful implementation of the GEL Initiative concept was a muscle-driven autonomous solution which employs a multi-farad electric double-layer capacitor (hecto- and kilofarad range capacitors are now available) as an intermediate energy storage to power a variety of portable electrical and electronic devices such as MP3 players, AM/FM radios, flashlights, cell phones, and emergency kits.[14] As the energy density of electric double-layer capacitors is bridging the gap with batteries, it is hoped that in the near future the automotive industry will start to deploy ultracapacitors as a replacement for chemical batteries.
Transportation applications
China is experimenting with a new form of electric bus (capabus) that runs without powerlines using power stored in large onboard electric double-layer capacitors, which are quickly recharged whenever the electric bus stops at any bus stop (under so-called electric umbrellas), and fully charged in the terminus. A few prototypes were being tested in Shanghai in early 2005. In 2006, two commercial bus routes began to use electric double-layer capacitor buses; one of them is route 11 in Shanghai. [15]
In 2001 and 2002, VAG, the public transport operator in Nuremberg, Germany tested a hybrid bus which uses a diesel-electric drive system with electric double-layer capacitors.[16]
Since 2003 Mannheim Stadtbahn in Mannheim, Germany has operated an LRV (light-rail vehicle) which uses electric double-layer capacitors to store braking energy.[17][18]
Other companies from the public transport manufacturing sector are developing electric double-layer capacitor technology: The Transportation Systems division of Siemens AG is developing a mobile energy storage based on double-layer capacitors called Sibac Energy Storage [19] and also Sitras SES, a stationary version integrated into the trackside power supply [20]. The company Cegelec is also developing an electric double-layer capacitor-based energy storage system[بحاجة لمصدر].
Proton Power Systems has created the world's first triple hybrid Forklift Truck, which uses fuel cells and batteries as primary energy storage and electric double-layer capacitors to supplement this energy storage solution.[21]
Technology
Graphene,Carbon nanotubes and certain conductive polymers, or carbon aerogels, are practical for supercapacitors:
- Graphene has excellent surface area per unit of gravimetric or volumetric densities, is highly conductive and can now be produced in various labs. It will not be long before large volumes of Graphene is produced for supercapacitors. For more details on this technology, refer to work of Prof. Rod Ruoff at Univ. of Texas.
- Carbon nanotubes have excellent nanoporosity properties, allowing tiny spaces for the polymer to sit in the tube and act as a dielectric. MIT's Laboratory of Electromagnetic and Electronic Systems (LEES) is researching using carbon nanotubes[22].
- Some polymers (eg. polyacenes) have a redox (reduction-oxidation) storage mechanism along with a high surface area.
- Supercapacitors are also being made of carbon aerogel. This is a unique material providing extremely high surface area of about 400-1000 m²/g. The electrodes of aerogel supercapacitors are usually made of non-woven paper made from carbon fibers and coated with organic aerogel, which then undergoes pyrolysis. The paper is a composite material where the carbon fibers provide structural integrity and the aerogel provides the required large surface. Small aerogel supercapacitors are being used as backup electricity storage in microelectronics, but applications for electric vehicles are expected. The voltage of an aerogel capacitor is limited to a few volts. Higher voltages will lead to ionization of the carbon, which will damage the capacitor. Carbon aerogel capacitors have achieved 325 J/g (90 Wh/Kg) energy density and 20 W/g power density.[23]
- The company Reticle claims to be able to make supercapacitors from activated carbon in solid form. This substance they call consolidated amorphous carbon (CAC). It can have a surface area exceeding 2800 m2/g and according to US patent 6787235 may be cheaper to produce than aerogel carbon.
- Systematic pore size control showed by Y-Carbon[24] can be used to increase the energy density by more than 100% than what is commercially available[بحاجة لمصدر].
- The company Tartu Technologies developed supercapacitors from mineral-based carbon. This nonactivated carbon is synthesised from the metal- or metalloid carbides, e.g. SiC, TiC, Al4C3, etc. as claimed in US patent 6602742 and WO patent 2005118471 . The synthesised nanostructured porous carbon, often called Carbide Derived Carbon (CDC), has a surface area of about 400 m²/g to 2000 m²/g with a specific capacitance of up to 100 F/mL (in organic electrolyte). They claim a supercapacitor with a volume of 135 mL and 200 g weight having 1.6 kF capacitance. The energy density is more than 47 kJ/L at 2.85 V and power density of over 20 W/g.[25]
In August 2007, a research team at RPI developed a paper battery with aligned carbon nanotubes, designed to function as both a lithium-ion battery and a supercapacitor (called bacitor), using an ionic liquid, essentially a liquid salt, as the electrolyte. The sheets can be rolled, twisted, folded, or cut into numerous shapes with no loss of integrity or efficiency, or stacked, like printer paper (or a Voltaic pile), to boost total output. Further, they can be made in a variety of sizes, from postage stamp to broadsheet. Their light weight and low cost make them attractive for portable electronics, aircraft, automobiles, and toys (such as model aircraft), while their ability to use electrolytes in blood make them potentially useful for medical devices such as pacemakers. In addition, they are biodegradable.[26]
انظر أيضاً
- Electric vehicle battery
- Capacitor
- Types of capacitors
- Nanoflower
- Rechargeable electricity storage system
- Flywheel energy storage
- List of emerging technologies
- Lithium ion capacitor
- Self Powered Equipment
- Nanocapacitor
المصادر
- ^ 5000F, Nesscap Products
- ^ http://www.act.jp/eng/index.htm
- ^ http://fplreflib.findlay.co.uk/articles/6610/if-the-cap-fits.pdf
- ^ المكثف الكهربائي مزدوج الطبقة, US DoE overview
- ^ http://fplreflib.findlay.co.uk/articles/6610/if-the-cap-fits.pdf
- ^ Researchers fired up over new battery, Deborah Halber, MIT News Office, February 8, 2006
- ^ http://www.act.jp/eng/index.htm
- ^ http://www.dailymotion.com/video/x65xr6_ultracapacitor-google-nbspvideo_tech
- ^ Carbon Nanotube Enhanced Ultracapacitors, MIT LEES ultracapacitor project
- ^ قالب:Ref patent
- ^ أ ب ت The Charge of the Ultra - Capacitors. IEEE Spectrum, November 2007
- ^ Высокоёмкие конденсаторы для 0,5 вольтовой наноэлектроники будущего
- ^ Prototype Test Results highly appreciated by Ultracapacitor Experts. APowerCap press release, 2006.
- ^ Muscle power drives battery-free electronics (Alexander Bell, EDN, 11/21/2005)
- ^ [1] (in Chinese, archived page)
- ^ VAG Verkehrs-AG Nürnberg
- ^ UltraCaps win out in energy storage. Richard Hope, Railway Gazette International July 2006
- ^ M. Steiner. MITRAC Energy Saver. Bombardier presentation (2006).
- ^ Siemens AG Sibac ES Sibac ES Product Page (as of November 2007)
- ^ Siemens AG Sitras SES Sitras SES Product Page (as of November 2007)
- ^ Proton Power Systems Unveils the World’s First Triple-hybrid Forklift Truck. Fuel Cell Works press release (2007).
- ^ MIT LEES on Batteries. MIT press release, 2006.
- ^ Lerner EJ, "Less is more with aerogels: A laboratory curiosity develops practical uses". The Industrial Physicist (2004)
- ^ Y-Carbon
- ^ Latest developments in carbide derived carbon (2006)
- ^ Beyond batteries: storing power in a sheet of paper. Rensselaer Polytechnic Institute press release (13 August 2007)
وصلات خارجية
- Super Capacitor Seminar
- Article on ultracapacitors at electronicdesign.com
- Article on ultracapacitors at batteryuniversity.com
- A new version of an old idea is threatening the battery industry (The Economist).
- An Encyclopedia Article From the Yeager center at CWRU.
- Ultracapacitors & Supercapacitors Forum
- Special Issue of Interface magazine on electrochemical capacitors
- Nanoflowers Improve Ultracapacitors: A novel design could boost energy storage (Technology Review) and Can nanoscopic meadows drive electric cars forward? (New Scientist)
- If the cap fits... How supercapacitors can help to solve power problems in portable products.