أرتزونات
Artesunate آرتيزيونات (INN) جزء منآرتيميزينين مجموعة من الأدوية لعلاج الملاريا . هو مشتق شبه مخلق من آرتيميزينين , وهو يذوب في الماء, ويمكن إعطاؤه عن طريق الحقن . و يشار اليه أحيانا بالإسم المختصرAS.
 | |
 | |
| البيانات السريرية | |
|---|---|
| النُطق | ahr-tez′ŭ-nāt[1] |
| الأسماء التجارية | many[2] |
| أسماء أخرى | SM-804 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| مسارات الدواء | By mouth, intravenous, intramuscular |
| فئة الدواء | Artemisinin |
| رمز ATC | |
| الحالة القانونية | |
| الحالة القانونية | |
| المعرفات | |
| رقم CAS |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.898 |
| Chemical and physical data | |
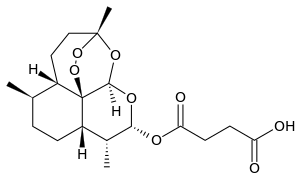
| التركيب | C19H28O8 |
| الكتلة المولية | 384٫43 g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الإستعمالات
آرتيزيونات يستحدم أساسا لعلاج الملاريا ولكن ظهر أخيرا قدرته على أن يكون أكثر فعالية بنسبة 90% في تقليل إنتاج البيض في عدوى Schistosoma haematobium .[4]
الجرعات
There are no licensed forms of artesunate available in the U.S. or UK. In the UK, artesunate is available on a named patient basis only.
Intravenous dose of IV artesunate:
- 2.4 mg/kg loading dose over 5 minutes
- 1.2 mg/kg dose 12 hours later
- 1.2 mg/kg once daily after that
Artesunate must always be given with another antimalarial such as mefloquine[5][6] or amodiaquine[7] so as to avoid the development of resistance. The combination of artesunate/amodiaquine has been found to be of equivalence to co-artemether.[8]
To obtain Artesunate, contact the CDC Malaria Hotline: 770-488-7788 (M-F, 8am-4:30pm, eastern time) or after hours, call 770-488-7100 and request to speak with a CDC Malaria Branch clinician.
التخليق
Artesunate is prepared from dihydroartemisinin (DHA) by reacting it with succinic acid anhydride in basic medium. Pyridine as base/solvent, sodium bicarbonate in chloroform and catalyst DMAP (N,N-dimethylaminopyridine) and triethylamine in 1,2-dichloroethane have been used, with yields of up to 100%. A large scale process involves treatment of DHA in dichloromethane with a mixture of pyridine, a catalytic amount of DMAP and succinic anhydride. The dichloromethane mixture is stirred for 6–9 h to get artesunate in quantitative yield. The product is further re-crystallized from dichloromethane. alpha-Artesunate is exclusively formed (m.p 135–137˚C).
المقاومة للدواء
Clinical evidence of drug resistance has appeared in Western Cambodia, where artemesinin monotherapy is common.[9] There are as yet no reports of resistance emerging elsewhere.
المراجع
- ^ "Artesunate definition". Drugs.com. Archived from the original on 20 December 2016. Retrieved 7 December 2016.
- ^ "Artesunate". Drugs.com. Archived from the original on 20 December 2016. Retrieved 7 December 2016.
- ^ "Artesunate for injection safely and effectively. See full prescribing information for Artesunate for injection. Artesunate for injection, for intravenous use Initial U.S. Approval: 2020". DailyMed. 5 November 2021. Retrieved 11 March 2022.
- ^ Boulangier D, Dieng Y, Cisse B; et al. "Antischistosomal إن فعالية تركيبات آرتيزيونات العلاجية المستخدمة كعلاج شافى لإصابات". Trans R Soc Trop Med Hyg. 101 (2): 113–16. doi:10.1016/j.trstmh.2006.03.003.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|الملاريا year=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Looareesuwan S, Viravan C, Vanijanonta S; et al. (1992). "Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria". Lancet. 339 (8797): 821–4. doi:10.1016/0140-6736(92)90276-9. PMID 1347854.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Nosten F, van Vugt M, Price R; et al. (2000). "Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study". Lancet. 356 (9226): 297–302. doi:10.1016/S0140-6736(00)02505-8. PMID 11071185.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Adjuik M, Agnamey P, Babiker A; et al. (2002). "Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial". Lancet. 359 (9315): 1365–72. doi:10.1016/S0140-6736(02)08348-4. PMID 11978332.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Meremikwu M, Alaribe A, Ejemot R; et al. (2006). "Artemether-lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trial". Malar J. 5: 43. doi:10.1186/1475-2875-5-43. PMID 16704735.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ White NJ (2008). "Qinghaosu (Artemisinin): The price of success". Science. 320 (5874): 330–334. PMID 18420924.